The VYRSA V1 is a fusion device that comprises a 3D-printed titanium body with a coarsened surface with pores, multiple openings, and anchor plates to be deployed into the ilium and sacrum to provide better fixation
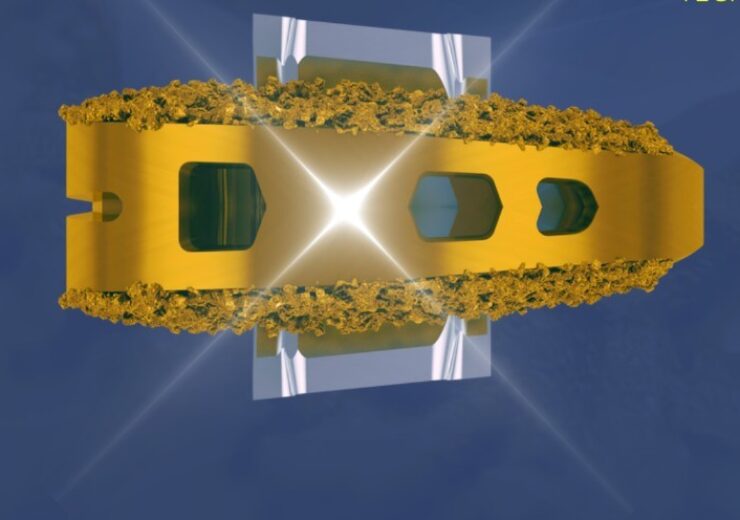
VYRSA V1 SI Fusion System. (Credit: VYRSA Technologies, Inc.)
US-based speciality medical device firm VYRSA Technologies has received the US Food and Drug Administration (FDA) approval for the VYRSA V1 SI Fusion System.
The VYRSA V1 is a fusion device that includes a 3D-printed titanium body with a coarsened surface that supports bone growth onto the surfaces of the device.
Its surface is designed with pores measuring about 500 microns in diameter, which is an optimal environment for the bone to grow and fully incorporate the implant within the sacroiliac joint.
VYRSA V1 comes with multiple openings, allowing a large volume of autogenous bone graft to integrate with the implant to enable fusion.
In addition, the device features two sharpened anchor plates within the 3D-printed body, which are deployed into the ilium and sacrum to provide better fixation.
VYRSA Technologies president Terry Harvey said: “The entry of the VYRSA V1 implant into the US market represents a huge step for VYRSA in continuing to provide innovative, physician-friendly solutions for the treatment of sacroiliac joint dysfunction.
“The FDA 510k clearance is a very exciting step forward for patients who suffer from pain due to sacroiliac joint disruptions and degenerative sacroiliitis.”
The FDA approval is based on comprehensive data submitted by VYRSA Technologies, from an extensive biomechanical testing protocol.
The testing protocol showed superior evidence of biomechanical stability in the sacroiliac joint after implantation with the VYRSA V1 SI fusion system.
In addition, data from the biomechanical testing reports for SI joint fixation showed that VYRSA V1 had no significant differences compared to control implant groups.
The testing found no statistically significant difference between the two techniques and is consistent with previous studies in the lateral and posterior approaches to SIJ fusion.
VYRSA Technologies is focused on surgical treatment technologies for musculoskeletal disorders and has a portfolio of MIS allograft implants and transfixing medical devices.
