The EUA facilitates to use of the instrument-free RT-PCR Covid-19 test for testing pooled patient samples in high-complexity CLIA-certified laboratories
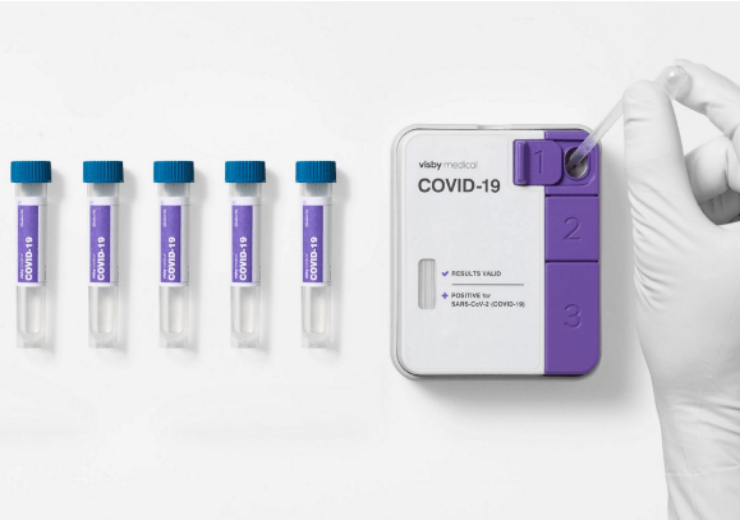
Visby’s Covid-19 test pooling protocol facilitates high-complexity clinical laboratories to combine up to five patient samples into a single device for processing. (Credit: Visby Medical, Inc.)
Diagnostics company Visby Medical has secured an emergency use authorisation (EUA) from the US Food and Drug Administration (FDA) for its Covid-19 reverse transcription (RT) polymerase chain reaction (PCR) for pooled samples.
The EUA facilitates to use of the instrument-free RT-PCR Covid-19 test for testing pooled patient samples in high-complexity clinical laboratory improvement amendments (CLIA)-certified laboratories.
The authorisation is also said to extend the earlier EUA for single patient sample testing at the point of care.
It also expands the capabilities of the company’s palm-sized PCR technology from individual, single-use testing to pooled testing of up to five patient samples at once.
Over 32 million COVID-19 tests were conducted in the US alone over the past month, according to the US Centres for Disease Control and Prevention (CDC).
Visby stated that pooling patient samples with its test will help increase overall lab testing capacity without additional tools or resources, while maintaining both accuracy and speed by returning results within 30 minutes.
Visby Medical medical affairs director Dr Teresa Abraham said: “With the SARS-CoV-2 virus ravaging the country, Visby remains steadfast in meeting the urgent needs of the community, its partners, and the changing market dynamics with our instrument-free PCR Covid-19 test.
“Thanks to this EUA, laboratories can meet increasing testing demand by analysing up to five patient samples at once, allowing for significantly increased efficiency and significantly decreased testing costs, particularly in low prevalence settings.”
Visby’s Covid-19 test pooling protocol facilitates high-complexity clinical laboratories to combine up to five patient samples into a single device for processing.
A negative result indicates that all five individuals have tested negative for SARS-CoV-2, said the company.
A positive result determines that all five samples are to be re-tested individually to confirm which patient or patients are infected.
The company’s platform provides quick and fast results by shrinking PCR technology to palm-sized dimensions. Visby’s single-use, the instrument-free device can rapidly detect Covid-19 infections.
In March this year, consumer health company Maverick Health collaborated with Visby Medical to provide rapid- Covid-19 PCR testing to its organisational partners.
