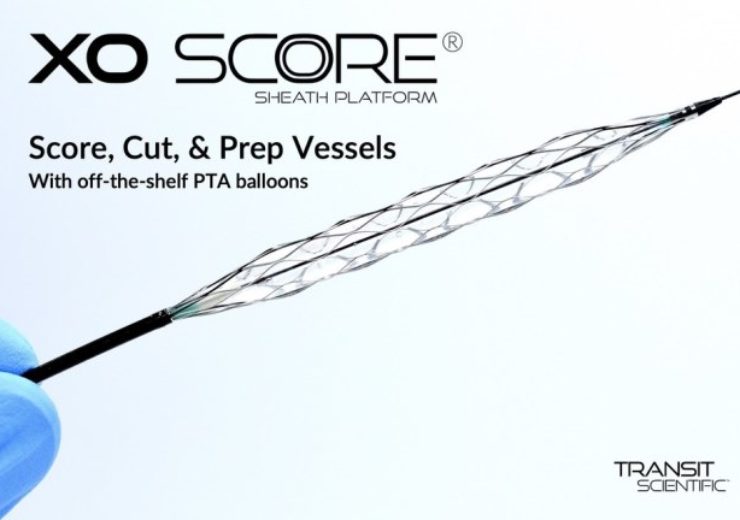
The XO Score is a patented and metal-alloy exoskeleton with a balloon-expandable scoring section
Transit Scientific has secured FDA approval for XO Score PTA scoring sheath platform. (Credit: PRNewswire / Transit Scientific)
Transit Scientific has secured approval from the US Food and Drug Administration (FDA) for its XO Score percutaneous transluminal angioplasty (PTA) scoring sheath platform.
The XO Score PTA scoring sheath platform is suitable for use in iliac, ilio-femoral, popliteal, infra-popliteal, and renal arterial plus synthetic or native arteriovenous hemodialysis fistula applications.
Angioplasty is conducted with expandable polymer balloon catheters for the dilation of stenosed, or narrowed vessels.
Calcified, fibrous, and/or resilient stenosis is said to need special scoring or cutting angioplasty balloons with integrated wires or blades on the balloon.
The new platform is a patented, low-profile, flexible, and metal-alloy exoskeleton with a balloon expandable scoring section.
One XO Score sheath can be used with multiple 4-8mm diameter and 20-40mm long balloons
One XO Score sheath can be used with multiple 4-8mm diameter and 20-40mm long balloons, enabling to save the hospital, outpatient lab, ambulatory surgery centre (ASC) or office-based lab (OBL) money on each scoring or cutting procedure.
The XO Score features up to 22 scoring/cutting struts, which lay flat while tracking but rotate 90° during balloon inflation to score and cut 0.25mm, 0.35mm or 0.50mm deep. Struts rotate 90° back during deflation.
The FDA clearance enables to cover the 6.3Fr (French) XO Score in 65cm and 125cm working lengths with 0.25mm, 0.35mm, or 0.50mm scoring or cutting depths to facilitate accurate dilation across a range of lesion types.
Transit Scientific is also focused on the development of 3Fr, 4Fr, and 5Fr over-the-wire and rapid-exchange XO Score versions for smaller vessel and coronary use.
In May, the company also secured FDA clearance for the XO Cross 2Fr, 2.6Fr, and 3.8Fr low-profile, high-torque, and high-push non-tapered microcatheter platform.
Transit Scientific president Transit Scientific said: “XO Score transforms regular PTA balloons into scoring and cutting systems.
“Clinicians insert an off-the-shelf PTA balloon into the XO Score tableside and then the system can be used over-the-wire to dilate calcified plaque and prep vessels.”
In April, Refine USA secured a class II medical clearance from the US Food and Drug Administration (FDA) for its medical grade microneedling device.
