The US FDA approval expands the use of MammoScreen from 2D to 3D mammography
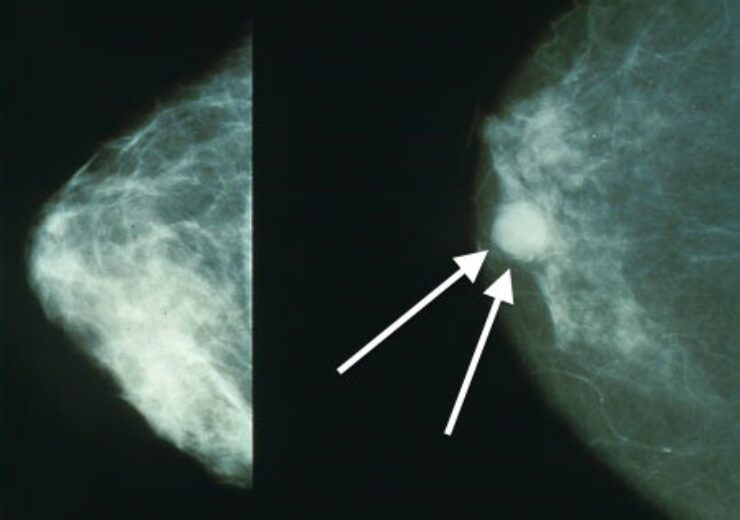
Normal (left) versus cancerous (right) mammography image. (Credit: Bakerstmd/Wikipedia.)
France-based Therapixel has received the US Food and Drug Administration (FDA) approval for MammoScreen, its AI-powered breast cancer screening software.
It is the second 510(k) approval from the FDA, and expands the use of its Mammoscreen to Digital Breast Tomosynthesis (3D Mammography), said the medical technology firm.
MammoScreen is a software that helps radiologists to improve their performance in interpretation of screening 2D and 3D mammograms.
The software automatically detects and characterises suspicious soft tissue lesions and calcifications in mammography and tomosynthesis images. It also evaluates mammography and tomosynthesis images to find any malignancy.
The tool summarises the results through MammoScreen Score, which characterises each lesion scored on a scale of 1-10, with 1, being least likely to reveal malignancy.
With the FDA approval, the Mammoscreen is enabled to be immediately commercialised across the US mammography market, said Therapixel.
Therapixel founder and chief scientific officer Pierre Fillard said: “Receiving this second FDA clearance for MammoScreen is a major milestone for Therapixel.
“Thanks to a deep and fruitful collaboration with radiologists, we have, over the last 18 months, turned the 2017 DREAM challenge winning 2D algorithm to a powerful FDA-cleared product for both 2D and 3D mammography.”
The FDA approval was based on the results from a multi-reader study conducted this year.
In the study, the AI-based software enabled the improvement in readers’ performance in screening for lesions, along with significant time saving, compared to radiologists alone.
According to the company, MammoScreen covers both the gold standard 2D mammography and the advanced 3D tomosynthesis modalities.
The tool received the CE mark approval in January this year.
Therapixel CEO Matthieu Leclerc-Chalvet said: “MammoScreen allows a more optimised and certain assessment by Radiologists and a speedier reassurance of women having breast cancer screening exams, resulting in a more efficient workflow and reduced costs for the healthcare system.
“Thanks to this new FDA clearance, we expect significant growth in the US market and we look forward to installing MammoScreen in additional radiology departments and institutions across the U.S. so that imaging professionals and women can benefit from its use.”
