The Ablamap® Software was used to detect sources of AF among these persistent/long-standing persistent AF patients who had symptomatic, recurrent AF despite at least 1 prior AF ablation procedure
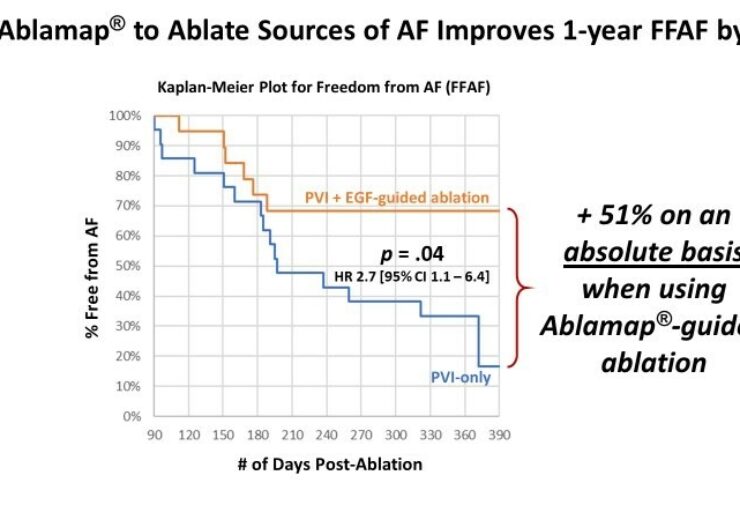
The FLOW-AF trial showed that ablating EGF sources improved freedom from AF at 1-year post-ablation by 51% on an absolute basis. (Credit: PR Newswire)
Ablacon, Inc., an Ajax Health and Zeus Health-backed developer of an innovative mapping system to guide the treatment of atrial fibrillation (AF), announced today the 12-month results from the FLOW-AF trial (NCT04473963). The data were presented by Dr. Vivek Reddy at the 2023 AF Symposium in Boston, MA at the Late Breaking Clinical Trials session. The trial enrolled 85 patients with persistent or long-standing persistent AF from 4 centers in the EU. The Ablamap Software was used to detect sources of AF among these persistent/long-standing persistent AF patients who had symptomatic, recurrent AF despite at least 1 prior AF ablation procedure. These patients were then randomized to pulmonary vein isolation (PVI) + EGF-guided source ablation v. PVI-only.
The FLOW-AF trial showed that ablating EGF sources improved freedom from AF at 1-year post-ablation by 51% on an absolute basis. Patients with an EGF-identified source left un-ablated had only a 17% rate of freedom from AF versus 68% rate of freedom from AF for patients who had their sources ablated which highlights the importance of phenotyping patients with EGF-identified sources as ablation targets. The trial also showed that EGF mapping allowed physicians to not only detect these important sources of AF and safely ablate them, but to also confirm their elimination with quick re-maps immediately after ablation.
“For the difficult-to-treat patients with persistent and long-standing persistent AF—and even more so for those undergoing repeat ablation procedures, Ablacon’s Ablamap technology gives physicians insight into the specific mechanisms of an individual patient’s AF disease rather than using traditional one-size-fits-all approaches,” said Dr. David Haines, who is a member of Ablacon’s Scientific Advisory Board and has used the technology in his own lab.
Ablacon is now preparing to run a large multinational pivotal trial. Duke Rohlen, CEO of Ablacon said, “We couldn’t be more excited about the results of FLOW-AF. Ablacon’s technology changes the treatment paradigm for AF by empowering us to ‘see’ AF — electrographic flow mapping focuses on understanding each patient’s underlying disease process to provide dynamic diagnostic and prognostic insights with actionable therapeutic targets.”
Source: Company Press Release
