Terumo has acquired 80.1% stake of Quirem Medical, making the company a wholly owned subsidiary of it
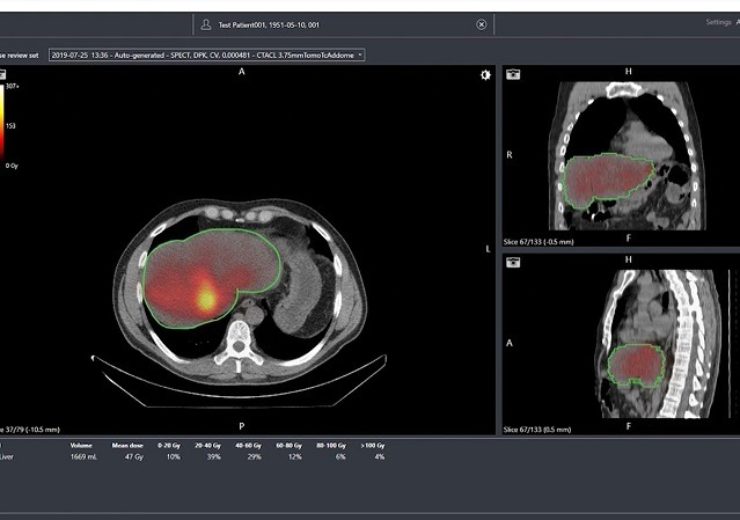
Q-Suite software, developed by Quirem Medical. (Credit: Terumo Corporation.)
Japanese medical technology company Terumo has closed the acquisition of Quirem Medical, a healthcare firm that develops advanced microspheres for Selective Internal Radiation Therapy (SIRT) for liver tumors.
Under the terms of the transaction agreement, Terumo has acquired 80.1% shares of Quirem Medical, making it the wholly owned subsidiary of Terumo.
Terumo will make an upfront payment of $20m, with an additional payment of up to $25m depending on the on the achievement of future milestones by 2030.
Terumo interventional systems division chief commercial officer Jim Rushworth said: “The acquisition of Quirem Medical further strengthens our business, expands on our manufacturing and clinical development activities, and complements our comprehensive suite of offerings to support our customers.”
The Netherlands-based healthcare firm offers QuiremSpheres, the only commercially available microspheres containing the radioactive isotope Holmium-166, whose safety and efficacy has been evaluated for the treatment of unresectable liver cancer.
The company said that its QuiremSpheres can be visualized and quantified even in low concentrations by means of Single-Photon Emission Computed Tomography (SPECT) and Magnetic Resonance Imaging (MRI), to improve patient selection, therapy planning and treatment verification.
Quirem Medical offers QuiremSpheres, QuiremScout and Q-Suite
Quirem Medical also provides QuiremScout, a low dose holmium microsphere that facilitates evaluation of microspheres before the therapy, and a dosimetry software package Q-Suite is used to plan QuiremSpheres treatments based on QuiremScout dose imaging.
Q-Suite is a software suit that is also capable of determining the SIRT success immediately after the procedure by converting SPECT and MR imaging into absorbed dose distributions.
Collectively, the three integrated products, QuiremSpheres, QuiremScout and Q-Suite constitute the Holmium SIRT platform, which helps physicians to optimise SIRT outcomes through a personalised treatment to meet the individual needs of each patient.
QuiremSpheres, QuiremScout and Q-Suite are CE Mark approved and currently available in Europe, the Middle East and Africa (EMEA).
Terumo is planning to commercialise the Holmium Platform across the world, in the coming years, as part of its ongoing expansion of its interventional oncology (IO) portfolio.
Terumo Europe global interventional oncology strategy and therapy development vice president Laurent Domas said: “By adding the Holmium-166 platform to our existing IO portfolio, we will further contribute to giving liver cancer patients a better future.
“This acquisition reflects Terumo’s commitment to build a broad platform of loco-regional treatment options for liver cancer and is another step forward as we aim to develop and provide treatment solutions for other organs as well.”
