The endovascular brain-computer interface (BCI) company will use the funding to support the development of its BCI product, initiate a clinical trial, and advance towards a BCI market approval for the treatment of paralysis
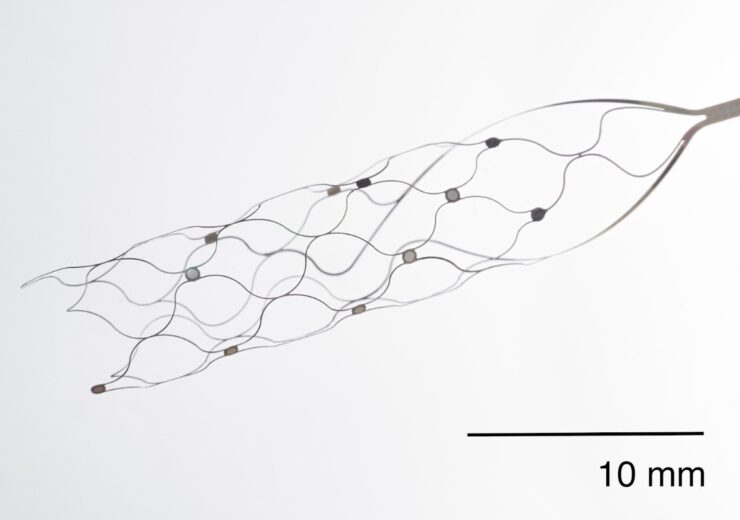
The Stentrode Endovascular Electrode Array. (Credit: Synchron)
Synchron, an endovascular brain-computer interface (BCI) company, has secured $75m in a Series C financing round led by ARCH Venture Partners.
The investment round has seen participation from existing investors, including Khosla Ventures, NeuroTechnology Investors, METIS, Forepont Capital Partners, ID8 Investments, Shanda Group and the University of Melbourne.
In addition, new investors Gates Frontier, Bezos Expeditions, Reliance Digital Health, Greenoaks, Alumni Ventures, Moore Strategic Ventures, and Project X have also participated in the financing round.
The Series C funding brings the company’s total investment to $145m.
Synchron will use the proceeds to advance the development of its first platform product, Synchron Switch BCI, and conduct its clinical trial.
ARCH Venture Partners co-founder and managing director Robert Nelsen will be appointed as Synchron’s Board Observer and Ari Nowacek as a member of the Board of Directors.
Robert Nelsen said: “At ARCH, our approach has always been to pair great science and technology with remarkable teams to build disruptive companies.
“The technology we witnessed at Synchron is helping people with previously untreatable conditions regain connection to the world. It is an exciting time for neurotechnology.”
Synchron is a clinical-stage endovascular BCI company that develops a BCI platform that eliminates the need for open brain surgery, using a minimally-invasive procedure.
Its Synchron Switch BCI is implanted in the blood vessel on the surface of the motor cortex of the brain through a minimally-invasive endovascular procedure via the jugular vein
Once implanted, the device will detect and wirelessly transmit motor intent to help severely paralysed patients control personal devices with hands-free point-and-click.
Synchron Switch BCI received the US FDA Breakthrough Device designation in August 2020 and an Investigational Device Exemption in July 2021.
The device is currently being studied in human clinical trials in the US and Australia.
Synchron founder and CEO Tom Oxley said: “We have an opportunity to deliver a first-in-class commercial BCI. The problem of paralysis is much larger than people realize. 100 million people worldwide have upper limb impairment.
“We are extremely excited to work with ARCH and this world-class syndicate to bring this technology to the people who need it.”
