The current FDA approval expands Explorer Air II’s indication to include its use with Pafolacianine, an optical imaging agent, to perform intraoperative fluorescence imaging in adult and paediatric patients aged one month and above
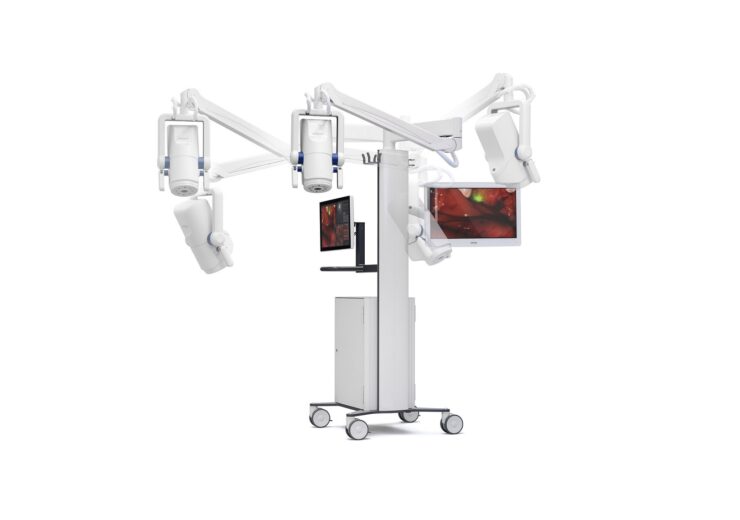
Explorer Air II is an intra-operative imaging system. (Credit: PRNewswire/SurgVision)
SurgVision, a subsidiary of the Bracco Group, has received the US Food and Drug Administration 510(k) approval for its Explorer Air II device.
The Explorer Air II is an intra-operative imaging system designed for in-vivo visualisation of fluorescence in the near-infrared range.
The system is already approved in the US and EU for visual assessment of blood flow and Indocyanine green (ICG) based tissue perfusion.
The current FDA approval expands its indication to include the use of Pafolacianine, an optical imaging agent, during intraoperative fluorescence imaging.
Explorer Air II can be used to perform intraoperative fluorescence imaging in adult and paediatric patients aged one month and above.
SurgVision CEO Stefan Schorling said: “We are very excited about this important milestone. Our goal is to make the Explorer Air II available to surgeons supporting them in their mission to fight cancer.”
The company said that the identification of tumours during surgery or endoscopy depends on visual inspection and palpation, and sometimes it is difficult to distinguish tumour tissue from healthy tissue.
SurgVision aims to develop highly sensitive imaging solutions to visualise tumours during surgical or interventional procedures in real-time, enabling accurate detection of the tumour.
The Dutch start-up said that its Explorer Air II is the first product developed based on its next-generation technology platform.
The imaging device is designed to deliver high sensitivity and imaging fidelity, meeting the needs of oncological intraoperative fluorescence imaging.
The company said the system facilitates real-time imaging during surgery, and its prototype has been tested by academic centres for several indications.
SurgVision is a medical technology company of Bracco Group, specialised in imaging technologies that enhance clinical practice, improve outcomes, and reduce the cost of care.
Based in Milan, Italy, Bracco Imaging, a part of the Bracco Group, is engaged in developing, manufacturing, and marketing diagnostic imaging agents and solutions.
The company offers a portfolio of products and solutions for all key diagnostic imaging modalities, including X-ray imaging, Magnetic Resonance Imaging (MRI), Contrast Enhanced Ultrasound (CEUS), and Nuclear Medicine.
