The new fully-endoscopic device allows easy integration of endoscopy during lumbar interbody fusion procedures with just one ultra-minimally invasive tubular retractor
OptiLIF Endo, featuring the Spineology OptiMesh Multiplanar Expandable Interbody Fusion System. (Credit: Business Wire/ Spineology Inc.)
Spine surgery technology solutions provider Spineology has announced the limited edition launch of its ultra-MIS system OptiLIF Endo for easy integration of endoscopy during lumbar interbody fusion procedures.
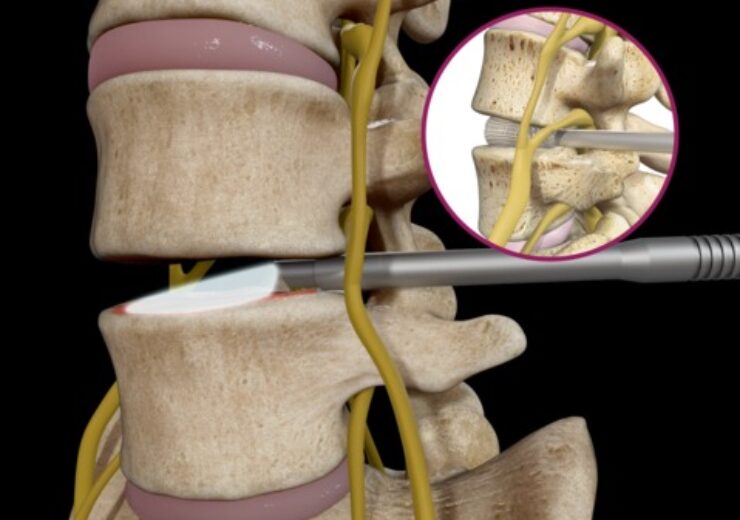
Spineology said the device requires only one tubular retractor to seamlessly integrate endoscopes and endoscopic equipment into lumbar interbody fusion procedures.
The single tube system uses the smallest diameter tubular retractor of any fusion system on the market due to the presence of OptiMesh Multiplanar Expandable Implant.
The procedures utilising OptiLIF Endo are done from beginning to end with just one ultra-minimally invasive tubular retractor in comparison to traditional MIS methods.
Spineology chief commercial officer Matt Cronin said: “OptiLIF Endo provides the power of direct endoscopic visualisation throughout the entirety of the procedure, including disc space access, decompression, discectomy/endplate preparation and interbody placement.
“Direct visualisation of the neural structures increases efficiency and safety when accessing the disc space, and the robust offering of new, endoscopic-specific discectomy tools included with OptiLIF Endo facilitates efficient endplate preparation under endoscopy to optimise fusion.”
The post-operative pain, blood loss, and duration of stay may all be positively impacted by such exposure reduction, which may also reduce collateral damage, said the company.
To maximise surgical efficiency and safety, OptiLIF Endo seamlessly transitions between the endoscopic and fusion equipment, reducing the need to change portal tubes or use numerous portal tubes at once.
Spineology CEO John Booth said: “I want to thank the surgeon development team and our engineers for developing such an innovative procedure and set of instrumentation that seamlessly integrates two powerful technologies.
“The endoscopic fusion market is gaining significant traction in the US as more surgeons see the value of incorporating endoscopic technologies into their procedures. With OptiLIF Endo, we will lead that market.”
In September last year, the company launched the Duo Ti Expandable Interbody Fusion Procedure.
