Duo system demonstrates reduced operative times, shorter hospital stays, and improved clinical outcomes compared to traditional lateral surgery
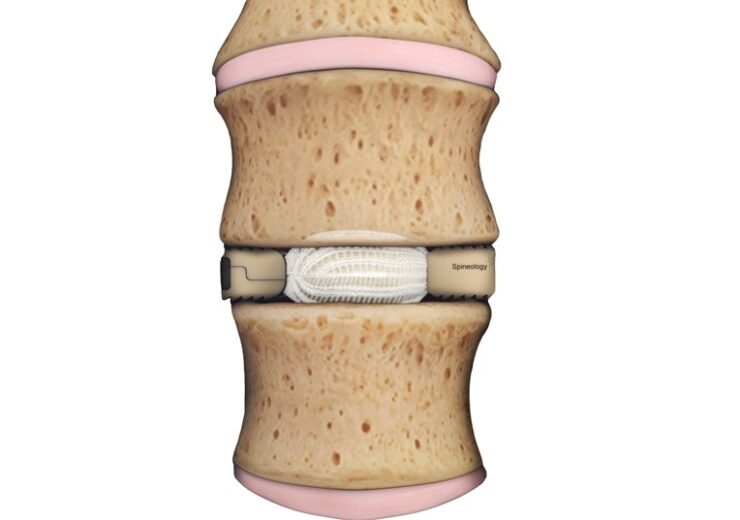
The Duo implant in the disc space (Credit: Business Wire)
Spineology, an innovator in anatomy-conserving spine surgery, is excited to announce full enrollment of its 200-patient Duo Expandable Interbody Fusion (IBF) System Post-Market Study, called RaDical. The RaDical Study is a prospective, Institutional Review Board (IRB)-approved, multi-center lateral interbody fusion study. It follows subjects for two years and measures intraoperative and postoperative metrics along with short- and long-term outcomes in a real-world patient population.
The Duo Expandable IBF System offers a unique implant design that is placed through an 18 mm portal tube. This minimizes retraction of the neural structures and psoas muscle to reduce or eliminate the neurological deficits commonly associated with traditional lateral interbody fusion procedures. The Duo implant is then filled with bone graft in situ to expand the device in all directions, providing a large, endplate-conforming, load-sharing interbody construct.
Data from the first 115 patients enrolled in the RaDical study demonstrated the Duo System’s benefits to the patient, surgeon and hospital when compared to traditional lateral interbody fusion systems, including:
– Reduced neurological deficits following surgery
– 33 percent decrease in total operative time
– 37 percent shorter hospital stay
– Lower complication rate
“The RaDical Study is one of the most innovative lateral studies ever conducted,” said study investigator Dr. Pierce Nunley, Shreveport, Louisiana. “We’re measuring subjective and objective data and looking at anterior thigh pain and weakness as early as two weeks post-op, which, to my knowledge, has not been done before. We have seen exciting results with the Duo System, including a very low onset of new neurological events, reduced surgical times, and shorter hospital stays.”
“We are extremely pleased to complete enrollment in the RaDical Study and see the positive impact of the Duo System design,” said John Booth, CEO, Spineology Inc. “Based on the data compiled to date, patients are experiencing long-term, clinically meaningful improvements in pain and function; high fusion rates; and fewer neurological deficits and complications compared to traditional lateral interbody fusion surgery. The data also shows a very positive economic impact to hospitals with shorter operative times and hospital stays compared to traditional lateral interbody fusion surgery. We look forward to collecting complete data on all 200 patients enrolled in the study and expect to see continued favorable outcomes, which will provide further evidence of the system’s benefits to patients, surgeons and hospitals.”
“My patients have had no neurologic issues post-operatively and far less pain,” added study investigator Dr. John Malloy, Coconut Creek, Florida. “This has resulted in shorter hospital stays and faster recovery.”
Source: Company Press Release
