Soliton has received approval of its laser for tattoo removal in May 2019, and is planning to file an additional 510(k) application for the use of RAP technology
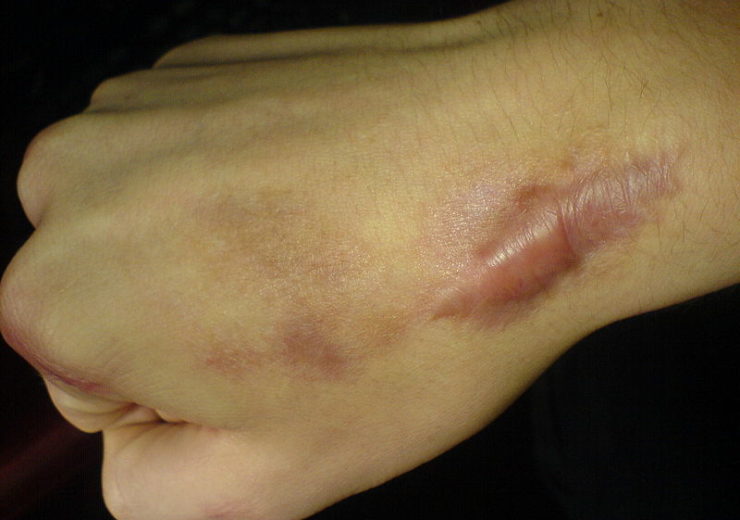
Image: Keloid formed after operation. Photo courtesy of Michael Rodger/Wikipedia.org.
US-based medical device company Soliton has announced its plans to conduct a proof of concept (POC) clinical trial for the use of its RAP technology for the treatment of keloid and hypertrophic scars.
Soliton is engaged in developing a unique platform technology licenced from the University of Texas on behalf of the MD Anderson Cancer Centre.
Keloid and hypertrophic scars occur due improper wound healing, and a post-surgical scar growing beyond its boundaries is a typical example of that condition.
According to the existing published research, the factors affecting wound-healing environment, including tension at the boundary of the scar, would cause fibrotic scars.
The fibroblasts are stuck in a hyper-productive loop and fail to stop production of collagen that leads to the thickened, raised and dense structures, which are often related to the fibrotic scars.
Soliton CEO, president and Co-founder Chris Capelli said: “Our preclinical studies combined with published literature on the behavior of fibrotic tissue have suggested that our acoustic shockwaves may be capable of disrupting stiff, sclerotic structures created by unwanted fibrosis, of which keloids and hypertrophic scars are just one example.
“Beyond this, we may also be able to help reset the targeted tissue to more normal fibroblast activity for lasting effects.”
According to American Osteopathic College of Dermatology’s estimation, the keloids affect approximately 10% of people, while hypertrophic scars are more common.
In addition, Keloid scars are more prevalent among populations with darker pigmentation, while Hypertrophic scars are found in men and women equally, irrespective of racial group, though 10-30 years old people are more likely to be affected.
Few treatment options are currently available for fibrotic scars
The company said that there are few treatment options available for fibrotic scars including surgical excision of the scar of corticosteroid injections, laser treatment or cryotherapy, which may cause significant discomfort, in addition to being disfiguring.
In addition, the direct injection of steroids into the scar is the most common treatment, which requires multiple injections and may not be a permanent solution. The radiation therapy would help prevent the return of the scar.
Soliton said that its device for use as an accessory to a 1064 Q switch laser for tattoo removal was approved in May 2019, and is planning to file an additional 510(k) application for the use of RAP technology. The technology for the treatment of cellulite and fibrotic scars is investigational and is not sold in the US.
