The pivotal cohort of the study featured patients having recurrent or refractory ascites caused by liver cirrhosis, who were implanted with the alfapump device
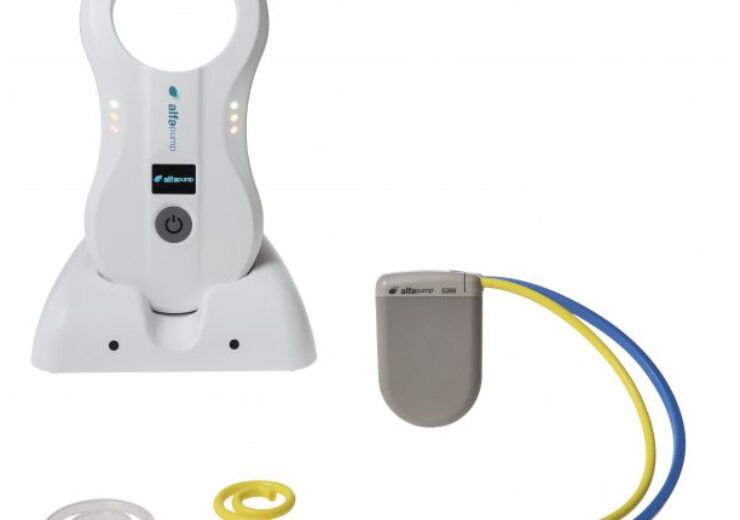
Alfapump - catheters and charger. (Credit: Sequana Medical NV)
Sequana Medical’s fully implantable alfapump device for the treatment of recurrent or refractory ascites due to liver cirrhosis has met all primary effectiveness endpoints in the pivotal cohort of the North American POSEIDON study.
According to Sequana Medical, data from 40 patients implanted with the device in the pivotal cohort showed statistical significance, while primary safety endpoint data was as expected.
The company expects the positive findings of the study to enable it in submitting a pre-market approval (PMA) application with the US Food and Drug Administration (FDA) in H2 2023.
Sequana Medical CEO Ian Crosbie said: “The third party market assessment highlights the large number of recurrent or refractory liver ascites patients, with strong forecast growth driven in part by the Non-Alcoholic Steatohepatitis (“NASH”) epidemic.
“We believe that there is a clear need for improved treatment options for this important patient group, and we are preparing to commercialize the alfapump through our focused commercial team.”
The pivotal cohort of the study involved patients having recurrent or refractory ascites caused by liver cirrhosis, who were implanted with the alfapump device. More than a third of the participants had NASH or combined NASH etiology.
One of the primary effectiveness endpoint hypotheses included median per-patient ratio of post-implant three-month observation period to the pre-implant three-month observation period pertaining to the number of therapeutic paracentesis (TP) being under 0.5.
Another primary effectiveness endpoint hypothesis is at least half of the patients achieving a 50% reduction in the requirement for TP in the same three-month period.
Of the patients implanted with the alfapump, 26 completed the therapy through day 180 post-implantation. These patients have a median reduction of 100% in frequency of TP in the post-implant observation period compared to pre-implant observation period.
Sequana Medical said that in the same period, 92% of patients have at least a 50% reduction in the number of TP.
The company revealed that other secondary efficacy and safety endpoints are being analysed and detailed findings from the POSEIDON study will be shared at an upcoming medical liver meeting in 2023.
