Cardiac Performance System is designed to use heart sound analysis for early detection and effective therapy guidance for patients suffering from heart failure and pulmonary hypertension
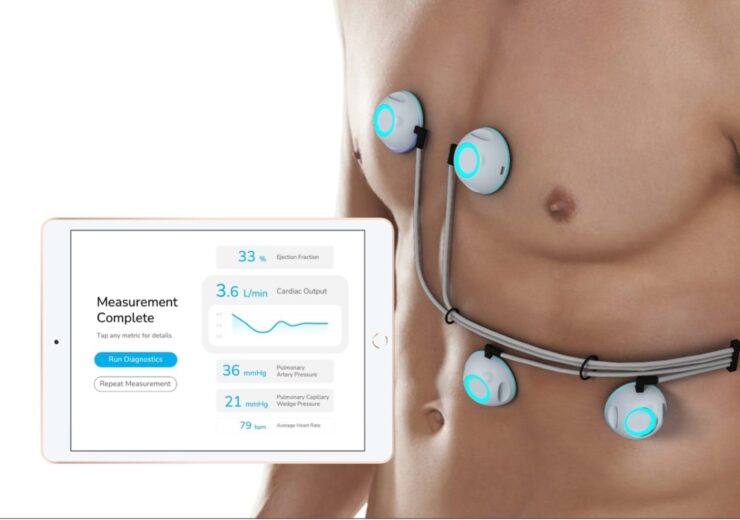
CPS is a non-invasive cardiac monitoring device. (Credit: Business Wire)
Cardiac assessment company Sensydia has concluded its 50-subject development study of its artificial intelligence (AI)-powered non-invasive Cardiac Performance System (CPS).
Cardiac Performance System is designed to use heart sound analysis for early detection and effective therapy guidance for patients suffering from heart failure and pulmonary hypertension.
It is said to deliver an accurate, non-invasive assessment of cardiac performance in less than five minutes, avoiding the need to visit a catheterisation lab.
The fifth trial of the cardiac monitoring device was conducted at the University of Minnesota (UMN).
The CPS platform is said to use ultra-sensitive biosensors to provide clinicians with fast, non-invasive measurements of ejection fraction, cardiac output, pulmonary artery pressure, and pulmonary capillary wedge pressure in a handheld device.
Currently, patients need to have invasive right cardiac catheterisation and echocardiogram to collect these parameters.
On the other hand, CPS assessments can be carried out practically any place with little training, quickly, safely, and as frequently as needed, Sensydia said.
Sensydia president and CEO Anthony Arnold said: “This is Sensydia’s fifth successful study, and we will continue to collect data across leading cardiac care institutions to improve the performance and utility of the artificial intelligence algorithms that power our breakthrough CPS platform.
“We’re working to overcome today’s barriers in acquiring vital hemodynamic measures for monitoring and managing cardiac patients, and we appreciate the participation of cardiologists and staff at University of Minnesota in working toward this goal.”
Sensydia previously carried out two studies at the University of Pittsburgh Medical Center for its pulmonary pressure algorithm and general clinical utility.
The cardiac assessment firm also completed one trial at the Ronald Reagan UCLA Medical Center that helped the US Food and Drug Administration (FDA) clear its ejection fraction algorithm.
The company also concluded one study at the OHSU Knight Cardiovascular Institute for its cardiac output algorithm.
In January 2022, Sensydia received the FDA breakthrough device designation for the CPS.
The cardiac assessment firm intends to create CPS pulmonary pressure algorithms using the data from this 50-subject trial.
