The FDA-cleared ultrasound system is based on Crystal Architecture to provide improved image clarity and penetration
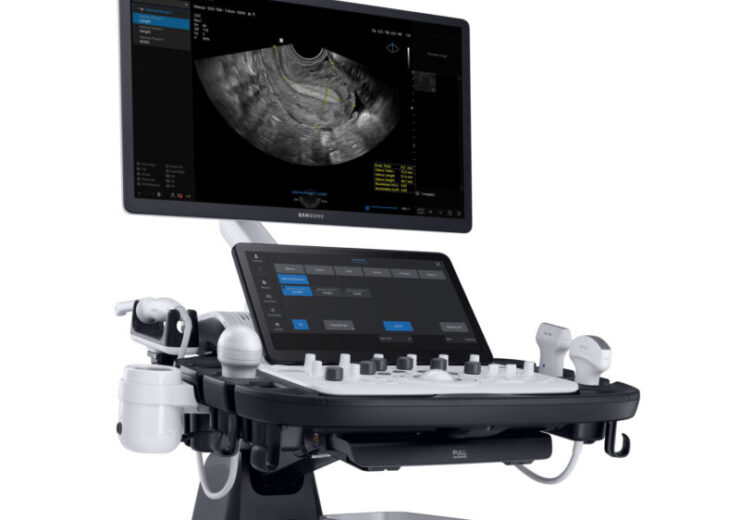
The V7 ultrasound system. (Credit: Business Wire)
Samsung Electronics’ US-based subsidiary Boston Imaging has unveiled a new ultrasound system called V7 with an aim to provide enhanced patient and user experience.
Recently, the ultrasound system secured FDA 510(k) clearance from the US Food and Drug Administration (FDA).
According to Boston Imaging, V7 is built to handle difficult clinical cases with accuracy and precision in a busy ultrasound setting as well.
Boston Imaging head David Legg said: “There is a continued demand on imaging professionals to deliver high-quality results in an efficient amount of time, without compromising patient care.
“We’re proud to help address this by supplying one more solution that helps make clinical assessments effortless and treatment precise.”
The new ultrasound system from the Samsung subsidiary is based on Crystal Architecture to offer improved image clarity and penetration.
Crystal Architecture is said to enable in producing clear, uniform, and high-resolution images by combining beamforming, sophisticated image processing, and S-Vue Single Crystal Transducers.
V7 is equipped with different Intelligent Assist features from Samsung, which allows it to be used in general imaging as well as ultrasound cases related to women’s health.
It has an automated 2D Follicle measurement tool that identifies and measures follicle size based on the 2D image. This lets the system generate information about the status of follicles during gynaecology examinations.
Boston Imaging has installed an AutoIMT screening tool on V7 to assess the potential risk of patients for cardiovascular disease.
Additionally, the ultrasound system incorporates the artificial intelligence (AI)-based UterineAssist technology that measures the uterus’ size and shapes automatically to help spot any abnormalities.
V7 is also integrated with the E-Cervix elastography technology to offer an efficient semi-quantitative evaluation of stiffness in the cervical canal, said Boston Imaging.
In April this year, Boston Imaging launched a new mobile digital radiography device called the GM85 Fit.
