The tool offers an on-device and computer-assisted detection (CADe) solution to identify pulmonary nodules from 10mm to 30mm in size
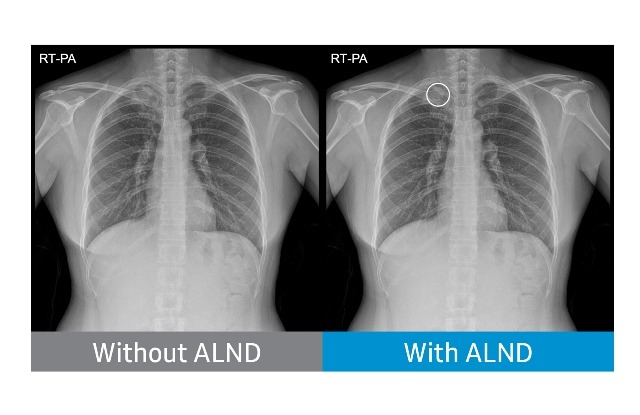
Chest radiograph without Auto Lung Nodule Detection (ALND) and chest radiograph with lung nodule marked. (Credit: Business Wire)
Samsung Electronics healthcare subsidiary NeuroLogica has secured 510(k) clearance from the US Food and Drug Administration (FDA) for its Auto Lung Nodule Detection (ALND) tool.
The new ALND tool offers an on-device and computer-assisted detection (CADe) solution to identify pulmonary nodules from 10mm to 30mm in size through an artificial intelligence (AI) algorithm.
NeuroLogica has developed the new tool to help physician to analyse PA chest radiographs of adults.
The ALND tool is part of S-Station, an operation software installed on Samsung Digital X-ray Imaging systems.
Samsung NeuroLogica digital radiography and ultrasound vice president David Legg said: “This FDA clearance is a huge milestone for Samsung and is the result of our tireless work to design diagnostic solutions that empower providers to deliver patients the absolute best care possible.
“The fact that it delivers clinically reliable results means clinicians can present it to patients with the utmost confidence, and for that we’re very proud.”
The new tool facilitates reader’s diagnosis by indicating the location of suspected lung nodules on chest X-ray images (posteroanterior chest radiographs).
According to the company, the deep-learning technology is clinically verified in multiple university hospitals, as well as approved with a sensitivity of 80% or more.
The tool also consists of an option (Autorun) to automatically perform nodule detection immediately after chest X-ray imaging. It also offers PACS transmission options to suit the hospital environment.
For accelerating diagnostic radiology using AI, Samsung is working with healthcare AI solutions developer Vuno.
Under the collaboration, the company will expand the uses of the chest CADe solution and enhance the diagnostic accuracy and workflow.
In July this year, NeuroLogica entered into a research collaboration with Massachusetts General Hospital to pilot OmniTom Elite system with Photon Counting Detector (PCD) technology.
