The Temporary Spur Stent System has been designed to treat long, diffuse and severely calcified infrapopliteal disease
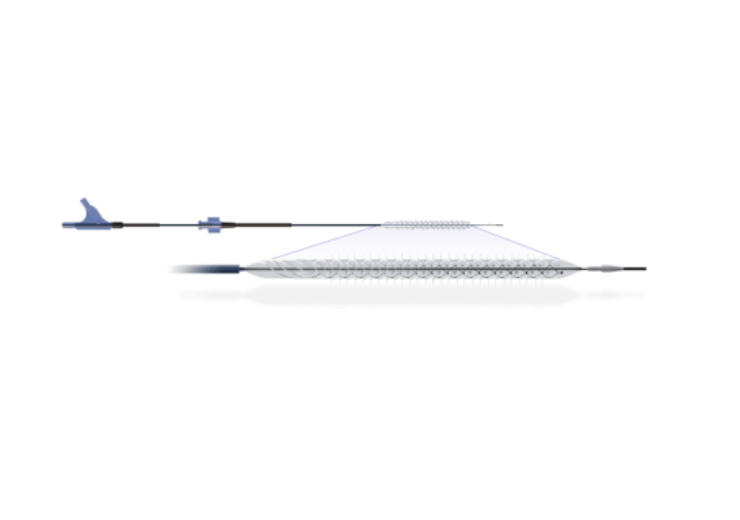
The Temporary Spur Stent System. (Credit: Reflow Medical, Inc.)
Medical devices maker Reflow Medical has recruited first patients in a non-randomised pilot study of the Temporary Spur Stent System.
The Temporary Spur Stent System is a novel retrievable stent designed for the treatment of long, diffuse and severely calcified infrapopliteal disease.
By using a LIMUS-base drug-coated balloon (DEEPER LIMUS), the first patients have been enrolled in a pilot study to treat lesions situated in the infrapopliteal arteries.
The trial principal investigator Dr Marianne Brodmann said: “This gives us an opportunity to truly understand how differently anti-restenotic drugs can treat infrapopliteal disease.
“The unique design of the Spur device may enable the anti-proliferative drug to reach the medial layers of the arterial wall, without leaving anything behind, and minimizing the need for follow-up procedures.”
Reflow Medical will recruit up to 30 participants in the single centre clinical study
Reflow Medical will recruit up to 30 participants in the OUS single centre clinical trial in around six months. The trial will follow primary and secondary endpoints for safety and efficacy.
Reflow Medical’s device is provided with a patented retrievable stent system that includes a series of radially expandable spikes designed to establish multiple pathways to facilitate the increased uptake of antiproliferative drugs into the vessel wall and enable acute luminal gain.
The company has designed the device to better treat patients suffering from critical limb ischemia (CLI), a serious condition in below-the-knee (BTK) disease that may cause high rates of restenosis and further treatment challenges.
Reflow Medical co-founder and CEO Isa Rizk said: “We are excited to gather data to support the clinical use of the Spur Stent in conjunction with a limus-coated balloon.
“This new study data will be added to a portfolio of other studies completed or currently enrolling on this groundbreaking medical technology.”
In January this year, Reflow Medical secured breakthrough device designation from the US Food and Drug Administration (FDA) for the Temporary Spur Stent System.
