Israel-based medical device company Rapid Medical has secured CE Mark approval for its Tigertriever 13, a fully visible clot retriever designed to treat ischemic stroke patients.
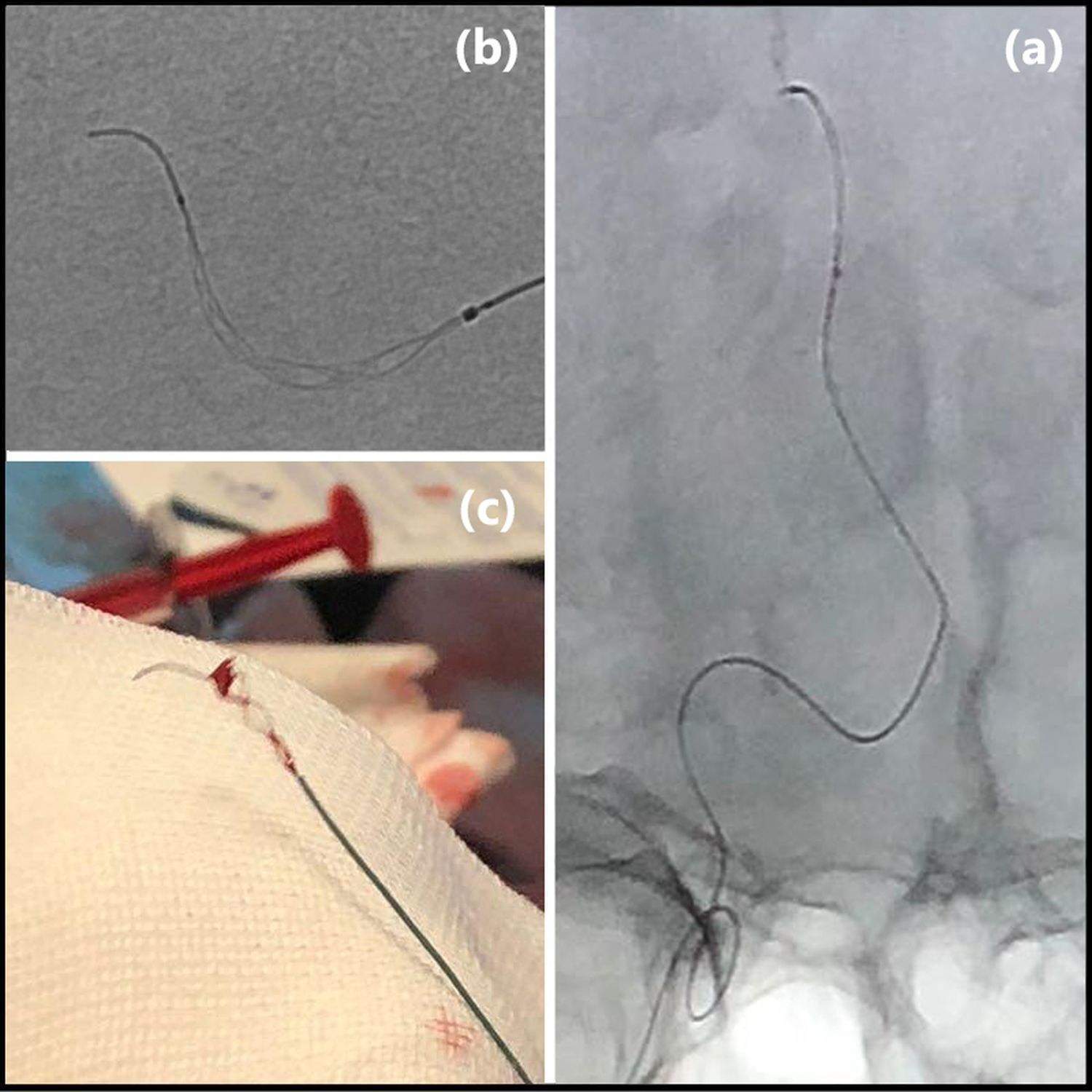
Image: TIGERTRIEVER 13 deployed in the distal Pericallosal artery (a). Angiographic image of the device (b). Image of the TIGERTRIEVER 13 with the retrieved clot (c). Images courtesy of Prof. Rene Chapot.
Rapid Medical is focused on the development of neurovascular devices.
The company’s Tigertriever 13 is claimed to have the smallest profile stentriever and is designed to safely retrieve clots from intracranial vessels between 1mm and 2.5mm.
Prof. René Chapot, Germany, said: “Tigertriever 13 is a very important addition to the ischemic stroke device market. For the first time ever, we have a tool that is dedicated to more distal occlusions. These occlusions can have a dramatic disabling effect on patients and until now there was little to be done for them. Using the Tigertriever 13 we were able to retrieve clots from an MVO that was not treatable until now.”
Nearly 1,500 patients have successfully been treated with the Tigertriever so far, stated the company. Its default profile is 83% smaller than any other device in the market and it is delivered through neurovascular microcatheter with a soft distal outer diameter of 1.3Fr.
The device has been designed to recanalise intracranial vessels between 1mm and 2.5mm. These medium vessel occlusions (MVO) may account for up to 30% of ischemic stroke patients and cannot be treated by any other device in the market.
This May, Rapid Medical started enrolling the first patients into TIGER (Treatment with Intent to Generate R eperfusion) study in the US.
There was a multi-center study of the performance of Tigertriever, the company’s thrombectomy device for the acute treatment of ischemic stroke.
The TIGER study is an IDE clinical study that will support the company’s 510(k) submission to the US Food and Drug Administration to obtain clearance to market the device in the US. It will take place in up to 25 leading stroke centers throughout the US.
