Epione allows clinicians to execute safe and efficient percutaneous tumour ablations, a minimally invasive procedure in which a needle is inserted through the skin to eliminate the tumour
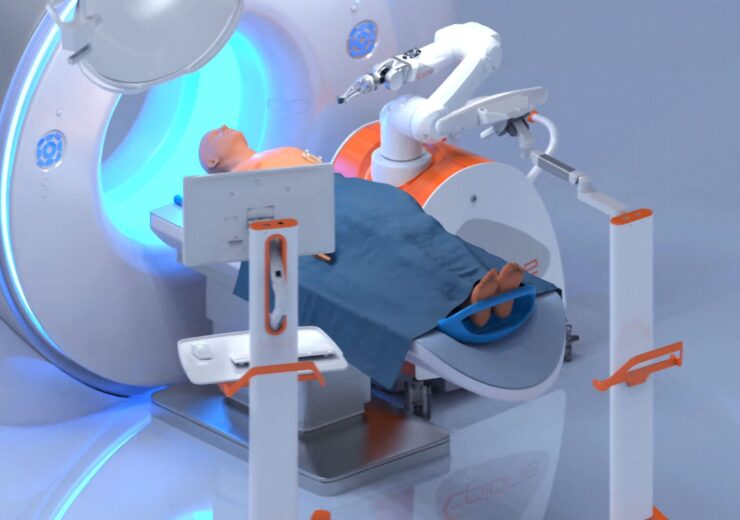
Epione, robot- assisted platform for cancer treatment. (Credit: Quantum Surgical)
French medical robotics company Quantum Surgical has received 510(k) approval from the US Food and Drug Administration (FDA) for its Epione, a robot-assisted cancer treatment technology.
The 510(k) clearance will allow the Epione interventional oncology robot to be sold and used commercially in the US to aid in the treatment of the primary causes of cancer death, early-stage liver disease.
Founded in 2017, Quantum Surgical developed the robotic-assisted platform to plan, target, administer, and validate tumour ablations.
Furthermore, Epione allows clinicians to execute safe and efficient percutaneous tumour ablations, a minimally invasive procedure in which a needle is inserted through the skin to eliminate the tumour.
Quantum Surgical president and co-founder Bertin Nahum said: “This clearance enables interventional oncologists to access state-of-the-art technology that has the potential to improve clinical outcomes and patients’ lives.
“We believe that the clinical adoption of innovative robotic solutions like Epione will be a significant step toward allowing more patients to benefit from minimally invasive therapies in cancer treatment.”
The company said more than three million people worldwide are diagnosed with the liver disease every year.
Quantum Surgical is planning to expand the platform’s range to more organs and add artificial intelligence-based decision-support functions.
The FDA approval is supported by the data provided by Prof. Thierry de Baere (Gustave Roussy Cancer Center, France) and Prof. Boris Guiu (Montpellier University Hospital, France) as their team employed the Epione system to treat primary and secondary liver cancers.
Stanford University Medical Center professor of radiology Nishita Kothary said: “It is tremendous to see Epione now available in the US as I believe technology like this will have a meaningful impact in the Interventional Oncology space.
“Percutaneous tumour ablation is a proven therapy that is underutilised today, and Epione will allow expansion of this minimally invasive treatment to more patients battling liver cancer.”
