SENTINEL is designed for capturing and eliminating embolic debris caused by transcatheter aortic valve replacement (TAVR) before it can reach the brain and potentially trigger a stroke
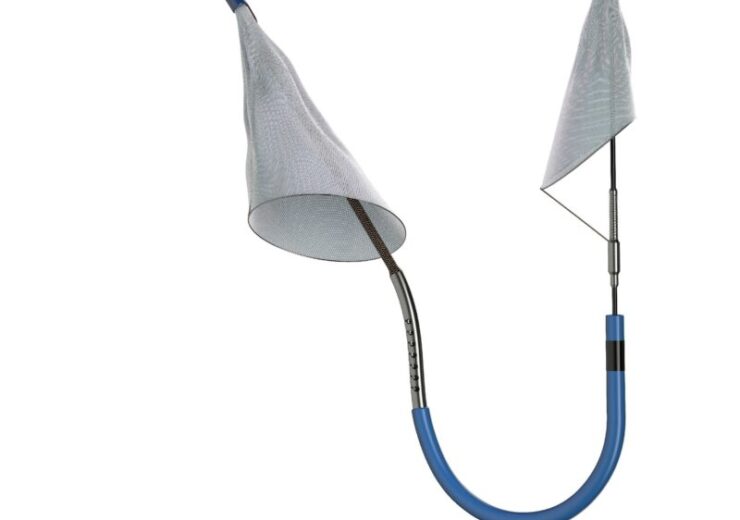
Boston Scientific's SENTINEL Cerebral Protection System. (Credit: Boston Scientific Corporation)
The PROTECTED TAVR clinical trial carried out by Boston Scientific to evaluate its SENTINEL Cerebral Protection System failed to meet the primary endpoint.
The SENTINEL device was shown in the trial to have cut down the risk of disabling stroke and low complication rate.
However, the data established a non-significant trend towards a lesser rate of stroke in patients treated with the device. This represented a relative risk reduction of 21% in all stroke through 72 hours or time of hospital discharge.
SENTINEL is designed for capturing and eliminating embolic debris stemming from transcatheter aortic valve replacement (TAVR) before it can reach the brain and possibly trigger a stroke.
Boston Scientific’s randomised trial studied reduction in periprocedural stroke and neurologic outcomes in patients having aortic stenosis who were either treated with the SENTINEL device to provide cerebral embolic protection (CEP) during TAVR or with only TAVR.
The company said that in a secondary analysis, a statistically significant 60% relative risk reduction was demonstrated in disabling stroke through 72 hours or time of hospital discharge in patients treated with SENTINEL.
Boston Scientific global chief medical officer Ian Meredith said: “Considering strokes are unpredictable, can occur regardless of an individual’s clinical background and often take a great toll on a patient’s quality-of-life and financial stability, we believe the data appear to demonstrate a consistent effect from CEP technology across all patient populations in the trial – supporting the use of the SENTINEL device as an effective therapy to reduce the risk of the most debilitating form of stroke for patients undergoing TAVR.
“We look forward to additional data on this technology such as from the currently enrolling PROTECT TAVI trial in the United Kingdom, which will similarly evaluate TAVR-related stroke reduction using the SENTINEL device.”
The PROTECTED TAVR clinical trial featured 3,000 patients spread over 50 global sites and at all surgical risk levels.
Before and after the procedure, all the patients in the trial had a neurological exam.
Boston Scientific said that analyses of subgroup showed that the reduction in disabling stroke with the use of the SENTINEL device was consistent throughout patient subgroups. These included age, gender, valve type, operative risk, as well as history of cardiovascular disease.
