The US-based medical device company is carrying out the A PRIORI study to assess the safety and performance of the Aortix pump to increase renal perfusion
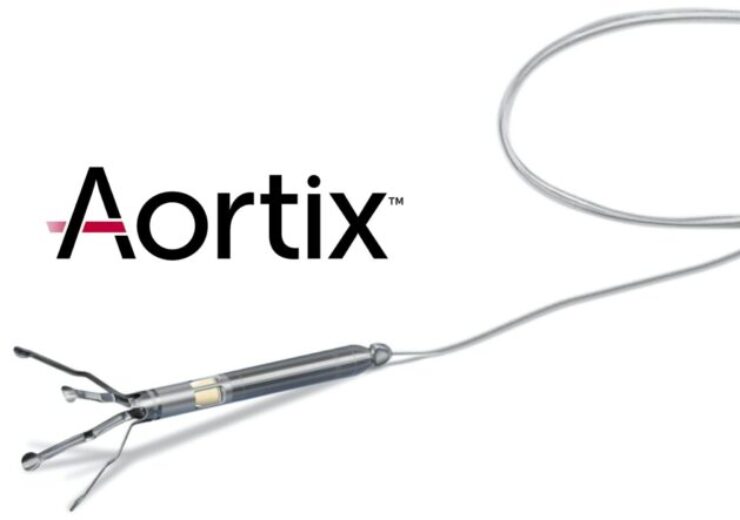
The Aortix percutaneous mechanical circulatory support device. (Credit: Procyrion)
Procyrion has reported the successful first-in-human (FIH) cases in the A PRIORI pilot trial of its Aortix percutaneous mechanical circulatory support (pMCS) device in the prevention of acute kidney injury (AKI) during cardiac surgery.
A PRIORI expands to Aortix therapy for Perioperative ReductIon Of Renal Injury.
The US-based medical device company is conducting the A PRIORI study to assess the safety and performance of the Aortix pump to increase renal perfusion.
The Aortix pMCS device is said to be a catheter-deployed pump technology that uses fluid entrainment to pump blood to address various conditions with unmet needs.
According to Procyrion, the A PRIORI study will enrol up to 50 patients across four clinical sites in Australia.
Procyrion president and CEO Eric Fain said: “The initiation of the A PRIORI study, after recently completing our pilot study in treating cardiorenal syndrome patients, is an exciting step that contributes to the growing body of evidence demonstrating the potential of the Aortix pump technology to improve outcomes for patients with cardiac and renal impairment.”
The Aortix pump is inserted into the descending thoracic aorta by a percutaneous catheter technique at the start of the scheduled surgical procedure, the medical device company said. This will directly boost kidney perfusion while decreasing cardiac energy requirements
As per Procyrion, intra-aortic implantation eliminates the risk of device-related thrombotic stroke or injury to the heart or valves, and the pump can be installed in less than 15 minutes.
The pump’s design uses fluid entrainment to provide therapy in a way that is physiologically natural while also directly increasing renal artery flow and pressure.
The Aortix pump should be used for up to 48 hours following surgery to maintain increased renal perfusion, the medical device firm added. This will provide extra protection against postoperative risk factors for AKI, and lessen the strain on the recuperating heart.
