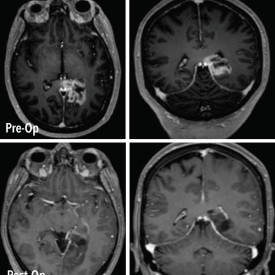
Pramand LLC announced today the US commercial launch of the CraniSeal Dural Sealant System. CraniSeal is indicated for use in patients ≥ 18 years of age as an adjunct to sutured dural repair during cranial surgery to provide watertight closure.
CraniSeal Dural Sealant is an absorbable polyethylene glycol (PEG) hydrogel that is applied with an applicator over sutures. This sealant prevents cerebrospinal fluid from leaking out of a cranial surgery incision site. The underlying technology has been used for two decades in a variety of medical applications.
Each year, approximately 160,000 cranial procedures are conducted in the US for reasons such as tumor resection, traumatic injury, and cerebrovascular disease. The volume of these procedures is growing by 3 – 5% annually. Cerebrospinal fluid leaks are one common complication of cranial surgery and can result in a longer hospital stay (+6 days) and higher medical costs (+$50,000).
Amar Sawhney, PhD, Pramand’s CEO, remarked, “We are excited to offer neurosurgeons and hospitals a choice for cranial dural sealing with CraniSeal which uses proven, highly effective technology with a long record of safety and efficacy.” Dr. Sawhney previously invented DuraSeal Dural Sealant, which has become the market-leading dural sealant globally.
The Pramand team achieved approval for CraniSeal, a Class 3 device that required Pre-Market Approval (PMA), in just 3.5 years. A typical Class 3 device takes 7 years to achieve approval through the PMA process.
Source: Company Press Release