TOMi Scope has been designed to determine the presence or absence of fluid in the middle ear
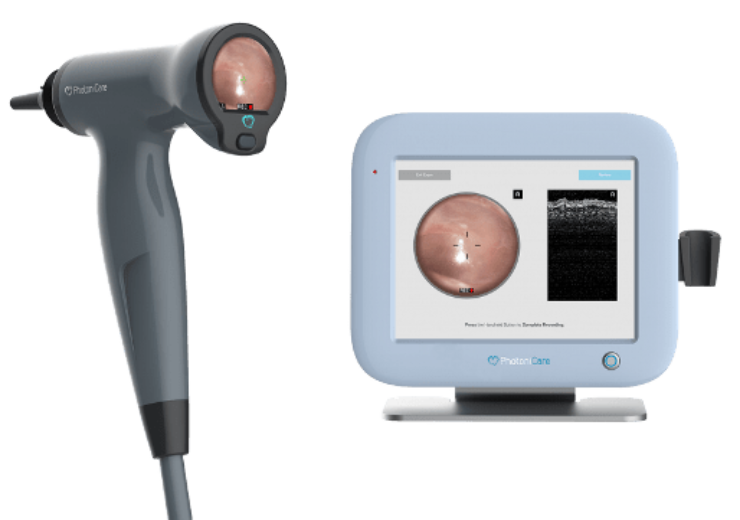
PhotoniCare's TOMi Scope for non-invasive imaging of the middle ear. (Credit: PhotoniCare, Inc.)
PhotoniCare has secured 510(k) clearance from the US Food & Drug Administration (FDA) for its TOMi Scope for non-invasive imaging of the middle ear.
By applying optical coherence tomography (OCT) high-resolution depth imaging, TOMi Scope confirms the presence or absence of fluid in the middle ear. It will also help in the characterisation of the fluid type.
TOMi Scope is claimed to be the first otoscope to provide non-invasive imaging of the middle ear
TOMi Scope is claimed to be the first and only otoscope to offer non-invasive imaging of the middle ear using a novel application of OCT to directly visualise and characterise fluid in the middle ear.
PhotoniCare’s TOMi Scope can be easily used by nurse-practitioner, physician’s assistant, or technician to conduct examinations accurately and efficiently.
It enables the physician to simultaneously assess the revealing OCT visual images of the middle ear while observing the otoscopic view of the eardrum surface.
Middle ear infections will lead to hearing loss, surgery and antibiotic use, specifically in children.
According to the company, children can go through recurring ear infections for six to 12 months before they are referred to an ENT specialist, and are repeatedly prescribed increasingly potent antibiotics during this time.
The company intends to immediately introduce the TOMi Scope in select US geographies, and the plans are underway for a full national launch later in 2020.
PhotoniCare co-founder and CEO Ryan Shelton said: “At PhotoniCare we set out to solve the massive problem of frequent misdiagnosis of middle ear infections, and the overuse of antibiotics and referrals to surgery in children that result.
“We thank the FDA for clearing our TOMi Scope under a new product code unique to our technology, and look forward to bringing this innovation to doctors and patients very soon.”
In May 2020, PhotoniCare announced that a multi-site registered clinical study was initiated to evaluate the imaging capabilities and image analysis performance of the TOMi Scope.
