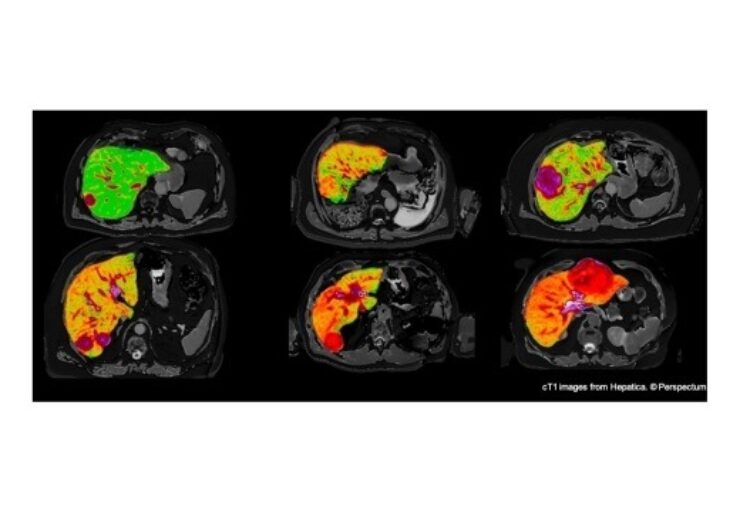
Hepatic uses non-invasive quantitative multiparametric MRI to provide a liver health assessment
Hepatica is Perspectum’s surgical decision support tool for liver cancer. (Credit: Business Wire)
Perspectum has secured 510(k) clearance from the US Food and Drug Administration (FDA) for its precision oncology decision support tool, dubbed Hepatica, for liver cancer.
According to the company, around 200,000 Americans are diagnosed with primary or secondary liver cancer every year.
Using non-invasive quantitative multiparametric MRI, Hepatica delivers a liver health assessment based on AI-driven liver segmentation along with proprietary biomarkers for hepatic fibro-inflammation and fat.
The application of AI to delineate the liver and calculate regional volumes will reduce pre-operative radiology time by more than 20 minutes in each case, said the company.
Hepatica enables to detect high risk patients
In a UK-based study on 143 surgical candidates, the initial results showed that Hepatica is able to efficiently detect patients at high risk of poor outcomes from surgery and a longer stay in hospital.
The demonstration included the aggregation of estimated future liver remnant with an assessment of underlying fibro-inflammation.
The information by the tool is expected to help tumour boards and multidisciplinary teams to take better pre-operative decisions to enhance post-operative outcomes for patients.
Perspectum said that Hepatica, which seamlessly incorporates into the clinical workflow, offers a simple and patient-friendly report through a cloud-based service.
San Diego’s University of California radiology professor Claude Sirlin said: “Hepatica is an advanced oncology tool that provides objective information to guide personalised clinical management.
“It is an excellent application of smart imaging and Artificial Intelligence in medicine, and I look forward to seeing how it can be used in a variety of indications to improve clinical care.”
In April 2020, Perspectum closed a $36m financing round, co-led by the Blue Venture Fund (BVF) and HealthQuest Capital.
The company decided to use the proceeds from funding to commercialise its first diagnostic product LiverMultiScan device for clinical use, expand the CRO business along with developing additional products for biliary disease, diabetes and cancer for both clinical and CRO applications.
