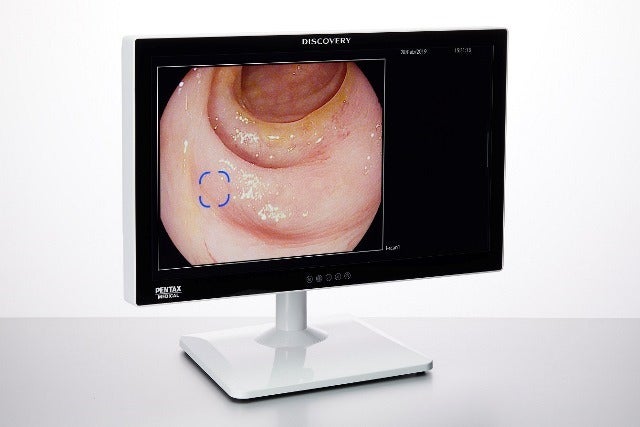
Hoya Group’s division Pentax Medical has secured CE mark for its Discovery artificial intelligence (AI) assisted polyp detector.
The company has designed the AI polyp detector to help endoscopists in detecting potential polyps during a colorectal examination.
Pentax Medical research centre, situated in Augsburg, of Germany, has worked with expert clinical partners from six of the major medical institutions across the world to develop Discovery AI-assisted polyp detector.
Over 120,000 files from around 300 clinical cases have been used for the software training to assist in the development of next-generation polyp detector.
Discovery AI assisted polyp detector will help clinicians to detect potential polyps in real-time.
Discovery can be used with any of Pentax Medical’s video endoscopy systems to show potential polyps
The new system is developed in a flat monitor to maximise usability. It can be used with any of the company’s video endoscopy systems to show potential polyps.
The menu, which is self-explaining, uses an advanced touchscreen interface. The company intends to introduce Discovery AI assisted polyp detector into the first markets in spring 2020.
Pentax Medical Augsburg managing director Dr Wolfgang Mayer said: “The benefits for the customers are outstanding. Our vision was to bring Artificial Intelligence into the operating room in the most user-friendly way.
“We wanted to give doctors the possibility to use this exciting new technology to strive for a better clinical outcome and maximize the patient care.”
In November this year, Pentax Medical introduced an advanced DEC HD duodenoscope in the US.
The high-definition duodenoscope includes multiple disposable components, including the sterile distal cap and elevator lever, for unit reprocessing.
Pentax has developed DEC HD Duodenoscope to offer physicians with a solution aligned with recent US Food and Drug Administration (FDA) guidance on decreasing endoscope reprocessing and deliver tangible “Triple Aim” value.
In August 2018, Pentax Medical purchased a controlling stake in French firm PlasmaBiotics for an undisclosed sum.



