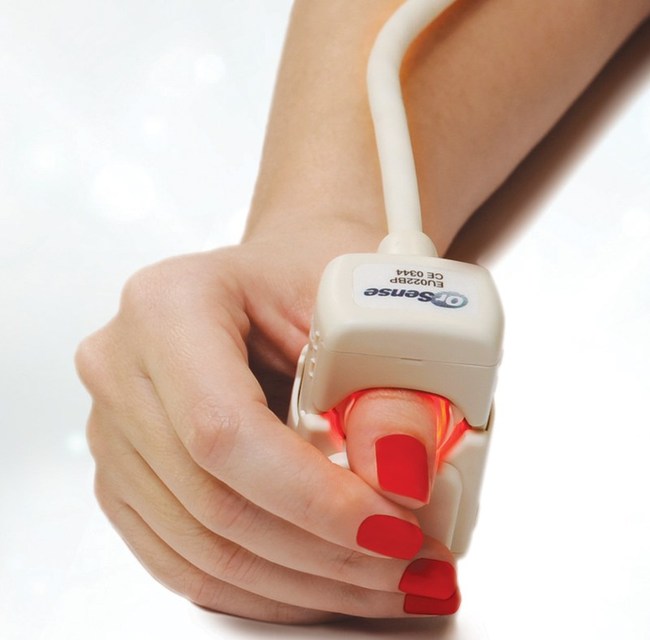
OrSense is a medical device company that develops and markets non-invasive hemoglobin, pulse, and oximetry measurement systems based on its innovative Occlusion Spectroscopy technology.
The OrSense NBM200 device provides painless, reliable and effective hemoglobin measurements, providing the mandatory pre-donation hemoglobin screening without traditional ‘finger prick’.
The CBER clearance from FDA is expected to permit full scale commercialization of the NBM200 System into all US blood collection centers.
The company said that the NBM200 marks the first ever non-invasive Hemoglobin measurement device to be cleared by the FDA/CBER for use in blood banks.
In addition, the same NBM200 System was previously cleared by the FDA/CDRH for use in professional healthcare markets including hospitals, physician offices, and public health departments for measuring hemoglobin, pulse, and blood oxygen levels.
OrSense US president Chip Neff said: “This new regulatory clearance will be welcome news for both blood donors and blood collection centers. The ‘finger stick’ is often mentioned by donors as the most painful part of the blood donation process.
“The OrSense NBM200 hemoglobin measurement is painless and represents a win/win for blood donors and blood bank organizations; it makes the blood donor experience more comfortable and provides for more efficient, lower cost operations for blood collection centers. The OrSense NBM200 will change the way pre-donation hemoglobin is measured by the U.S. blood bank industry.”
OrSense claims that its NBM200 device has been used since 2014 by blood bank and plasma collection centers across the world, to screen millions of donors.
OneBlood, one of the largest blood collection organizations in the US, has been using the NBM200 device for over two years under FDA special permit.
In July 2014, OrSense announced the first delivery of its NBM200 non-invasive Hemoglobin testing systems in Brazil, following the recent approval of the NBM200 by Anvisa, the Brazilian Health Surveillance Agency.



