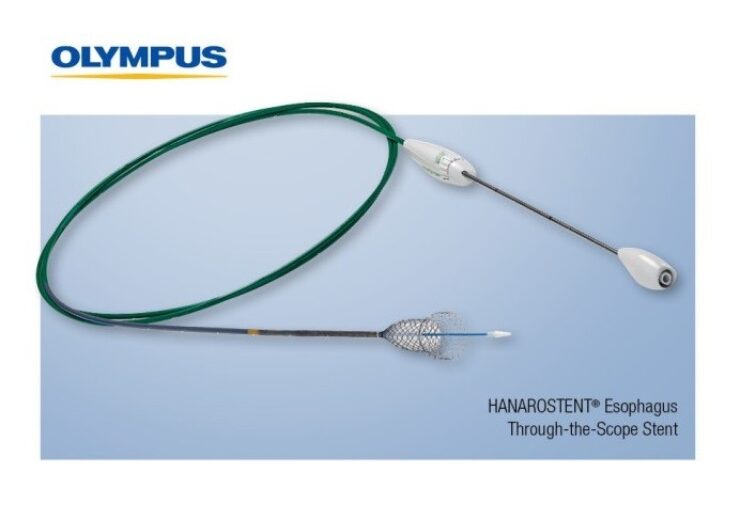
The Through-the-Scope (TTS) esophageal stent, the newest addition to the Olympus self-expanding stent portfolio, helps to achieve luminal patency in a variety of clinical applications and is designed for use in palliative treatment of esophageal stricture and/or trachea-esophageal fistula caused by malignant tumors
Olympus announces full commercial launch of HANAROSTENT Esophagus TTS stent. (Credit: PRNewswire / Olympus Medical Systems Group)
Olympus, a global technology leader in designing and delivering innovative solutions for medical and surgical procedures, announced today the commercial launch of HANAROSTENT Esophagus TTS self-expanding metal stents (SEMS) made by M.I. Tech and distributed exclusively through Olympus in the U.S.
The Through-the-Scope (TTS) esophageal stent, the newest addition to the Olympus self-expanding stent portfolio, helps to achieve luminal patency in a variety of clinical applications and is designed for use in palliative treatment of esophageal stricture and/or trachea-esophageal fistula caused by malignant tumors. The stent offers a fully covered or partially covered option.
“From the first cases in which I’ve used it, I found the HANAROSTENT Esophagus TTS stent to effectively palliate the symptoms of our patients with advanced esophageal cancer,” said Dr. Sandeep Patel, a gastroenterologist at UT Health San Antonio. “The hook-cross nitinol design of the TTS stents allows for accurate and efficient placement with increased patient comfort.”
Pre-loaded into the delivery system, the HANAROSTENT Esophagus TTS fits down the working channel of a standard therapeutic gastroscope and is deployed for placement in the esophagus. The hook-and-cross nitinol design, unique to HANAROSTENT products, was designed with patient comfort in mind and enables optimal radial and axial force allowing for the flexibility to conform to a patient’s anatomy and precisely target the stricture.
Unlike over-the-wire esophageal stents, the new 10.5F HANAROSTENT Esophagus TTS allows for accurate and easy esophageal stenting under direct endoscopic visualization. Additional benefits of the new esophageal stent include:
Minimized Use of Fluoroscopy: Direct visualization of the lumen allows physicians to place the stent with reduced dependence on fluoroscopy.
Increased Procedural Efficiency: Through-the-Scope application allows for fewer scope/device exchanges, leading to increased efficiency and potential reduction of procedure time.
Unique Wire Structure: Hook-and-cross nitinol design provides optimal radial and axial force aimed to minimize the impact of retrosternal pressure, which can be painful to patients.
Intuitive Delivery System: Long delivery length options, ability to fully recapture stent up to a point-of-no-return, as well as a retrieval lasso for stent repositioning combine to allow for safety and precision of placement for users of all experience levels.
“We are pleased with the positive clinical feedback we have received from our trusted physician customers since our limited launch of HANAROSTENT Esophagus TTS in July,” said Kevin Mancini, Vice President for Endoscopy at Olympus America Inc. “We are confident the HANAROSTENT Esophagus TTS will help our physician customers address quality of life issues in patients suffering from advanced gastrointestinal disease.”
“Through our valued partnership with Olympus, the HANAROSTENT Esophagus TTS is the sixth stent successfully launched in the U.S. market in the past two years,” said Jin-Hyung Park, CEO at M.I. Tech Co. Ltd. “We are thrilled to continue expanding capabilities of physicians in the U.S. and across the world, through the combination of a line of unparalleled stents and the trusted Olympus brand.”
Source: Company Press Release
