The company secured 510(k) clearance from the US Food and Drug Administration (FDA) for Excellagen for the management of wounds
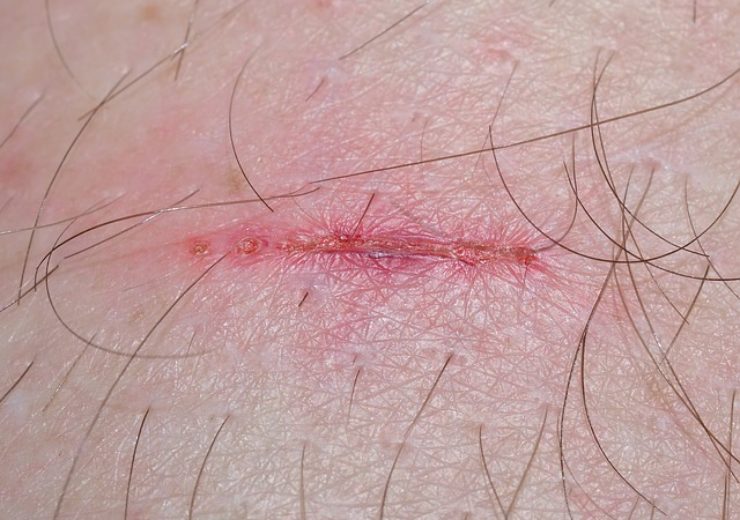
Excellagen Aesthetics facilitates a favourable wound healing environment (Credit: Pixabay/sipa)
Olaregen Therapeutix, a subsidiary of Generex Biotechnology, has introduced a new product called Excellagen Aesthetics for the cosmetic surgery and aesthetic dermatology market.
The company secured 510(k) clearance from the US Food and Drug Administration (FDA) for Excellagen for the management of wounds.
Excellagen can be used in facial rejuvenation procedures, including post-laser surgery, post-chemical peels, and post- skin ablation. It is a ready-to-use three-dimensional wound conforming matrix, which facilitates a favourable wound healing environment.
Excellagen activates collagen, enhance granulation and boost new tissue growth
The new product will help activate collagen, enhance granulation and boost new tissue growth by offering a structural scaffold for cellular migration and proliferation.
Olaregen’s Excellagen has been demonstrated in vitro to facilitate the localised release of endogenous growth factors such as Platelet-Derived Growth Factor (PDGF), a major biological mediator of wound healing.
With the support of contract sales force, Olaregen is launching the three-dimensional wound conforming matrix in major metropolitan areas across the US.
Olaregen business development and sales senior vice president Scott Emmens said: “We have been working closely with the aesthetic dermatology community, and Excellagen Aesthetics is being tested with some leading dermatologists who are conducting case studies to evaluate the efficacy of our cellular tissue product in wound management as measured by patient reported down-time as well as patient satisfaction with post-treatment care.
“We are enthusiastic about the early response from patients and doctors who have tried the product after facial rejuvenation procedures including micro-needling and laser skin resurfacing, two procedures that result in post-treatment pain and significant healing times that limit daily activities.”
Generex Biotechnology is an integrated healthcare holding company, which offers end-to-end solutions ranging from rapid diagnosis to personalised therapies for patient-centric care.
Olaregen, a regenerative medicine company, is involved in the development, manufacturing and commercialisation of products for the wound care market.
In July 2019, Generex Biotechnology acquired Medisource Partners, an FDA-registered distributor of medical and surgical products.
