Pulse is designed to integrate multiple technologies into one platform, to address most common clinical challenges in spine surgery
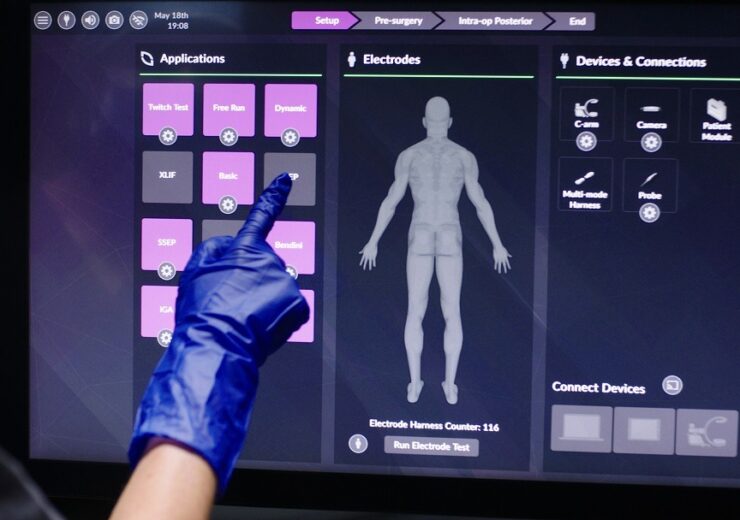
Pulse allows surgeons to easily access multiple technologies. (Credit: NuVasive.)
US-based medical devices company NuVasive has received the Food and Drug Administration (FDA) 510(k) approval for its Pulse platform for spine surgeries.
The company has also announced the commercial launch of the platform in targeted global territories.
The Pulse platform has already received CE mark approval for commercialisation and clinical use in Europe, in June this year.
Pulse is an integrated multiple technologies platform, designed to enhance safety, effectiveness, and procedural reproducibility of spine surgery.
The platform enables surgeons to easily access multiple technologies from a compact footprint and addresses few most common surgical challenges.
It is developed to reduce operating room (OR) time, lessen time under anaesthesia and intraoperative risks, and decrease with length of stay in the hospital.
NuVasive chief executive officer Christopher Barry said: “The Pulse platform launch is an incredible milestone for NuVasive and will help lead the digital transformation of spine surgery.
“Surgeons are now able to seamlessly work with various technologies to address more clinical challenges in surgery from a single platform—something they could not do before Pulse.
“This is the culmination of years of research and development to deliver a platform that helps improve clinical, financial, and operational outcomes.
“Like we did with XLIF, Pulse is a disruptive technology that has the ability to transform not only the trajectory of NuVasive but the future of spine care for patients.”
With the integration of various applications, Pulse facilitates less invasive and more advanced surgical procedures, providing benefits to the patient, surgeon, and hospital.
The platform’s extensible architecture is expected to support future applications, including robotics and smart tools.
It is claimed to support numerous imaging systems and provide enhanced integration with Siemens’ 3D mobile C-arm—the Cios Spin.
In addition, the Pulse platform introduces a procedurally integrated navigation technology for improved screw placement accuracy and minimise radiation.
NuVasive global business units executive vice president Massimo Calafiore said: “This first-of-its-kind platform supports all spine procedure types from open to less invasive techniques.
“Pulse is one of the most versatile tools in the spine OR, and the integration of multiple technologies in one platform enhances a surgeon’s capabilities to make better, more informed clinical decisions for their patients.
“I want to thank our NuVasive team and the many surgeons who helped bring Pulse to market and enable better spine surgery.”
