The partnership will develop a Covid-19 serological test that can be used in different health care settings
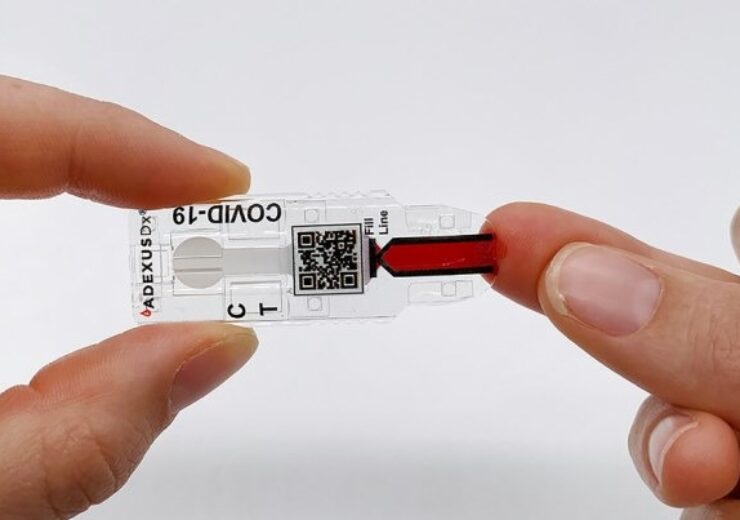
The ADEXUSDx Covid-19 test is a rapid serology and self-contained assay that measures the presence of SARS-CoV-2 antibodies. (Credit: NOWDiagnostics, Inc)
NOWDiagnostics has collaborated with the US Department of Health and Human Services’ Biomedical Advanced Research and Development Authority (BARDA) for the development of a rapid Covid-19 antibody test.
The partnership will engage in the development of a serological test for SARS-CoV-2 antibodies.
The tests will be developed for use in different health care settings ranging from clinics to hospital emergency rooms and by consumers at home.
The rapid Covid-19 antibody test uses the self-contained ADEXUSDx platform provided by NOWDiagnostics. It does not need any additional materials such as reagents and buffers, equipment, processing, or refrigeration.
ADEXUSDx Covid-19 test will offer lab-quality results in 15 minutes
The ADEXUSDx Covid-19 test, which will provide lab-quality results in 15 minutes, helps to identify the presence of SARS-CoV-2 antibodies in individuals who were exposed to the virus.
The test uses only a fingerstick to draw 40μL of capillary blood, venous whole blood, serum, or plasma at homes and workplaces.
In May 2020, the company also filed an application with the US Food and Drug Administration (FDA) for moderate complexity use of the ADEXUSDx Covid-19 test.
According to NOWDiagnostics, the point-of-care (POC) and over-the-counter (OTC) tests will help easily and rapidly screen individuals, as well as deliver results in minutes.
In June, Empatica has partnered with the BARDA to validate a wearable system that offers early warning for Covid-19 and other respiratory infections.
The partnership is aimed at validating Empatica’s algorithm in real-life settings, involving healthcare workers exposed to a high viral load while treating hospitalised Covid-19 patients.
Tn February 2019, Empatica and BARDA’s Division of Research, Innovation and Ventures (DRIVe) started the development of a digital biomarker to predict respiratory infections.
