The HFX spinal cord stimulation (SCS) system from US-based Nevro has received the CE mark for painful diabetic neuropathy (PDN) and non-surgical back pain (NSBP)
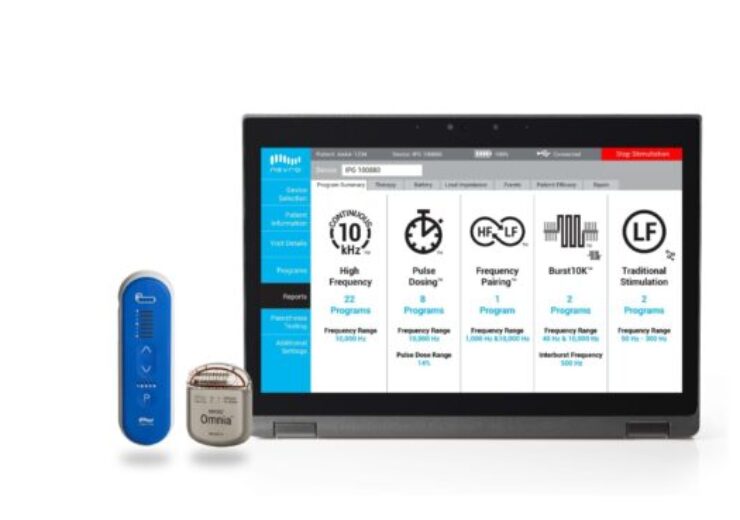
A image of Omnia driven by HFX Connect. (Credit: PRNewswire/ Nevro Corp.)
US-based medical device company Nevro has launched HFX Connect in Europe and received the CE marking of expanded labelling for the HFX spinal cord stimulation (SCS) system.
The HFX SCS system has received the CE mark for painful diabetic neuropathy (PDN) and non-surgical back pain (NSBP).
According to Nevro, the recent developments are intended to deliver solutions to treat chronic pain.
The solutions also enable greater healthcare system efficiencies, patient access to remote optimisation, and labelling for outcomes other than pain relief.
The medical device company said that the HFX Connect will be available to both newly implanted Omnia patients and those who already have the device. This will enable medical staff to remotely optimise patients using 35 pre-programmed, customisable treatment plans and seven times the amount originally available.
The recent regulatory approval also contains increased HFX labelling. HFX is now said to be the first and only SCS therapy in the world with a CE mark that has been approved for PDN patients’ pain alleviation and sensory improvement.
All the primary SCS indications, including back and leg pain, non-surgical back pain, painful diabetic neuropathy, and upper limb and neck pain, are covered by the recent specific labelling, Nevro said.
Additionally, the labelling now consists of anticipated clinical advantages besides pain alleviation.
Nevro CMO David Caraway said: “This development marks a significant milestone for Nevro’s commitment to improving patients’ access to HFX Therapy and delivers on the promise of upgradability we made when we launched the Omnia platform.
“For over a decade, Nevro has collected unparalleled outcomes data from over 100,000 implanted patients in the HFX Cloud and analysed how multiple programmes improve patient outcomes in different chronic pain types.
“All of this has been in preparation for the development of our data-driven HFX Algorithm and our innovative product pipeline, from which HFX Connect is a great example.”
The commercial launch of HFX Connect in Europe comes after a successful commercial debut in the US and Australia.
In October last year, Nevro obtained the US Food and Drug Administration (FDA) approval for its Senza HFX iQ SCS system.
