The NAUTILUS study is structured to evaluate the safety and effectiveness of the RNS System, which deliver personalised treatment targeting the source of the seizure, to treat generalised epilepsy in 12 years and older patients
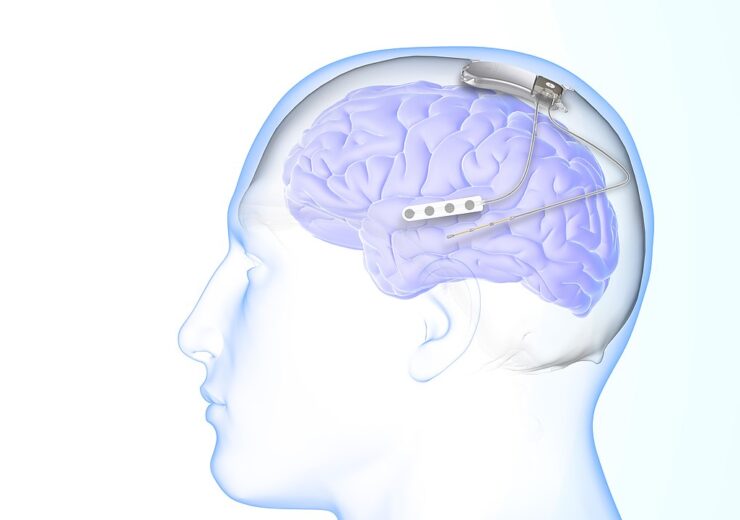
The NeuroPace RNS System for epileptic seizures. (Credit: ArinaGr19/Wikipedia)
US-based medical device company NeuroPace has announced the implantation of the first patient with its RNS System in the NAUTILUS clinical study.
NAUTILUS is designed to evaluate the safety and effectiveness of the RNS System in people aged 12 and above, with drug-resistant idiopathic generalised epilepsy (IGE).
The RNS System is a neurostimulation device, approved by the US Food and Drug Administration (FDA) for the treatment of drug-resistant epilepsy.
The system continuously monitors the brain activity and identifies a unique seizure pattern, to deliver personalised treatment targeting the source of the seizure, said the company.
The first procedure using RNS System was conducted at Vanderbilt Health in Nashville, Tennesse, by the study co-investigators Angela Crudele, and Dario Englot.
Dr Crudele said: “Today, we have limited treatment options for patients who have drug-resistant, idiopathic generalised epilepsy. This condition can be very difficult to treat and has a significant impact on a patient’s and family’s quality of life.
“I am excited about the possibility of having an FDA-approved treatment for this population, such as a brain-responsive neuromodulation, and giving these patients a better future.”
According to the company, its RNS System is the only FDA-approved brain-responsive neuromodulation system that delivers personalised treatment for seizures.
It deploys a closed-loop technology that monitors and responds to a patient’s unique brain patterns, and delivers therapy in real-time, prior to the onset of symptoms.
Last year, NeuroPace secured the FDA Breakthrough Device Designation status for its RNS System to treat idiopathic generalised epilepsy.
NeuroPace chief medical officer Martha Morrell said: “This is a groundbreaking study that could allow individuals who have drug-resistant generalised epilepsy to be treated with the RNS System.
“Brain-responsive neuromodulation is a proven therapy for drug-resistant focal epilepsy, with long-term studies demonstrating significant seizure reduction and quality of life improvements for patients.
“I look forward to investigating whether this therapy could provide similar benefits to patients suffering from primary generalised epilepsy, helping to fill a large unmet need in this population.”
