Relivion, a non-invasive digital medical device, is used for the treatment of migraine and depression
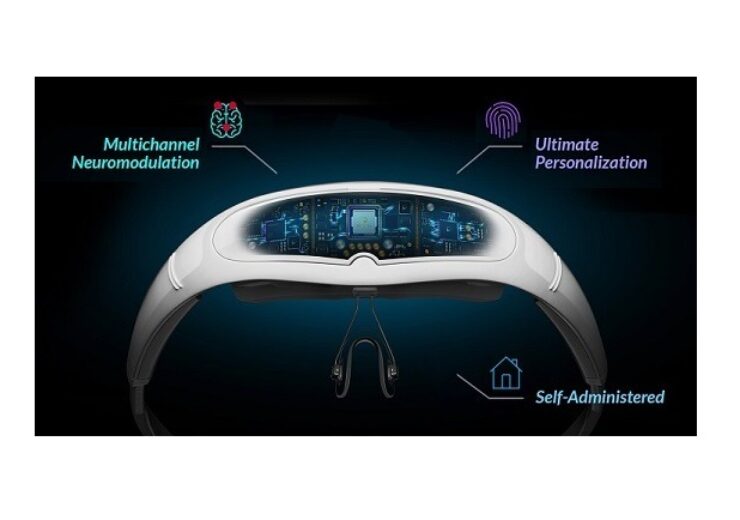
Neurolief and Sawai have collaborated to develop and commercialise neuromodulation device called Relivion in Japan. (Credit: Sawai Pharmaceutical Co.,Ltd)
Neurotechnology company Neurolief has signed an exclusive agreement with Sawai Pharmaceutical to develop and market a brain neuromodulation device called Relivion in Japan.
Relivion is a non-invasive digital medical device designed for the treatment of migraine and depression.
The medical device is placed around the head to deliver unparalleled stimulation to the occipital and trigeminal nerves.
The stimulation produces cumulative effect by releasing neurotransmitters in the brainstem as well as modulating brain networks associated with the control of pain and mood.
Relivion allows at home use, and patients will be able to share treatment data with their physician. Through a dedicated app, patients are also allowed to upload the data to the cloud database.
Relivion helps to analyse treatments using AI technology
The device also facilitates self-learn and analyse the treatments using AI technology, thereby helping to offer better treatments for patients as per their symptoms.
With an aim to launch Relivion in Japan, the company plans to submit applications to the Pharmaceuticals and Medical Devices Agency (PMDA) for the device to treat migraine by 2022, as well as depression by 2023.
Relivion is said to become the only brain neuromodulation device available for treatment at home in Japan, if the device is approved by the agency.
Neurolief already secured CE mark approval in Europe for Relivion to treat migraine and started preparations to launch the device in the European market.
In November 2020, the company completed a 510(K) submission with the US Food and Drug Administration (FDA) for the device to treat migraine. It is also planning to submit an application with the FDA for depression by 2022.
In August 2020, Neurolief secured breakthrough device designation from the FDA for its Relivion DP system to treat major depression.
