MindsEye Port is designed for the treatment of stroke, cancer, and other conditions
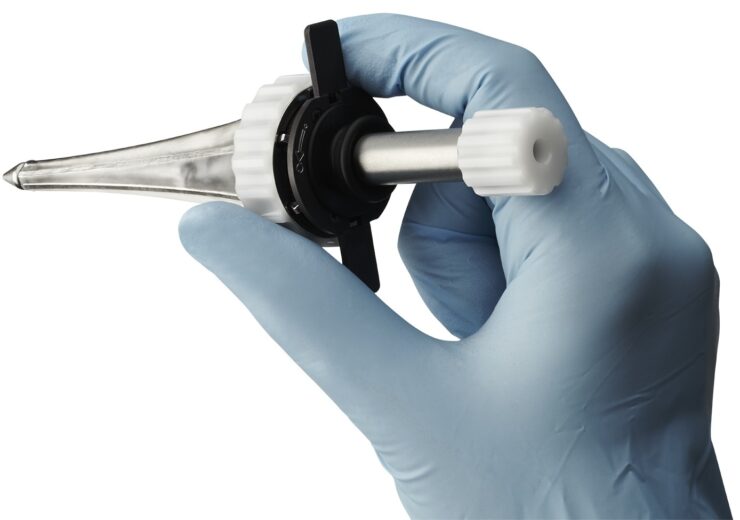
Minnetronix Medical received FDA approval for MindsEye Port for neurosurgical procedures. (Credit: Business Wire.)
Minnetronix Medical has received the US Food and Drug Administration (FDA) approval for its first platform product, dubbed MindsEye Expandable Port for neurosurgical procedures.
The company said that its MindsEye Port is designed for the treatment of stroke, cancer, and other conditions, and is a minimally invasive, expandable port.
With its dynamic retraction feature that creates a custom-sized channel, the device allows surgeons to reach target areas deep within the brain.
University of Cincinnati Medical Centre neurosurgery professor Mario Zuccarello said: “This is next-generation deep brain access technology. Minimizing invasiveness as much as possible is important to respect the eloquence of brain tissue.
“The MindsEye Expandable Port allows surgeons to work without unnecessary distractions, which ultimately improves quality of life for the patient. The port represents an entire platform for the company, as it has additional applications and complimentary product opportunities in the neuro market and beyond.
Minnetronix expands its traditional offerings to include market-ready platforms
Established in 1996, Minnetronix Medical is engaged in providing design, development and manufacturing services for medical device companies around the world.
With the FDA approval for MindsEye Port, the Saint Paul, Minnesota-based medical device manufacturer expands its traditional offerings to include market-ready platforms.
Furthermore, the company is currently developing a portfolio of solutions aimed at advancing the treatment options, prevent secondary injury and enhance healing for patients in the Neuro ICU.
Minnetronix Medical vice president and general manager Matt Adams said: “Given its unique features, the port has already attracted interest among neurosurgeons and potential distribution partners. They’re excited to get their hands on it.
“The MindsEye Port is an exciting example of how Minnetronix Medical is expanding its offerings by developing market-ready products.”
In May, Minnetronix featured among the eight US manufacturers selected by NASA’s Jet Propulsion Laboratory (JPL) in Southern California to produce its new ventilator, customised for coronavirus (Covid-19) patients.
