The platform will initially provide a robotically assisted total knee replacement solution that is compatible with the Evolution Medial-Pivot Total Knee System
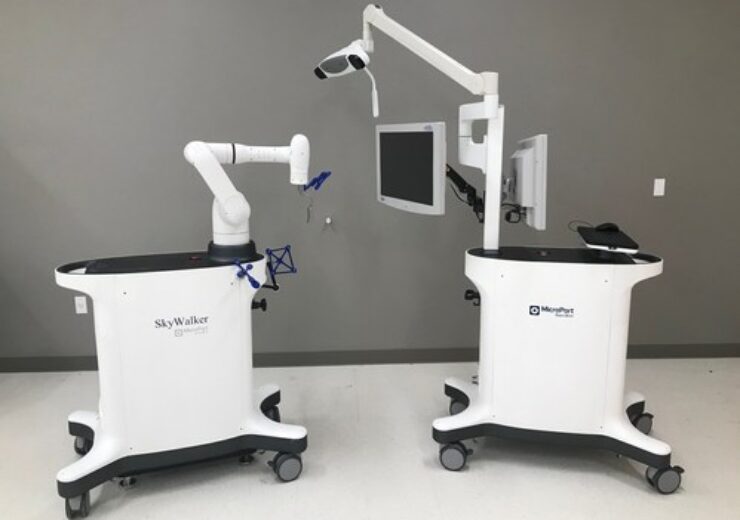
The SkyWalker System. (Credit: PR Newswire/ MicroPort Orthopedics)
MicroPort Navibot has secured the US Food and Drug Administration (FDA) 510(K) clearance for its first robot-assisted platform for orthopaedic applications, SkyWalker System.
The platform will initially provide a robotically assisted total knee replacement solution that is compatible with the Evolution Medial-Pivot Total Knee System.
According to the firm, the SkyWalker System prioritises safety while having the technological advantages of accurate operation and effective coordination.
The planning system helps surgeons create individualised patient implant plans based on preoperative CT scan anatomical data as well as certain implant data before the actual surgery.
The system enables precise implant location during surgery based on actual patient alignment and anatomy to obtain the appropriate kinematics unique to the patient.
Leveraging a high dexterity and light mechanical arm, the SkyWalker System then enables the surgeon to quickly go on to resection, improving precision, accuracy, and efficiency.
The SkyWalker System can give the surgeon information that can aid in achieving the desired joint line restoration while also providing information for the best possible soft tissue balancing.
The firm plans to work with MicroPort Orthopaedics to develop other orthopaedic applications for a more complete orthopaedic solution in the near future.
MicroPort NaviBot, which was created based on clinical requirements, has constructed a full technology research facility with medical industry design capabilities.
