The company designed AccuPlate based on the patient's scanned anatomical data, and are milled out of pure, implant-grade titanium
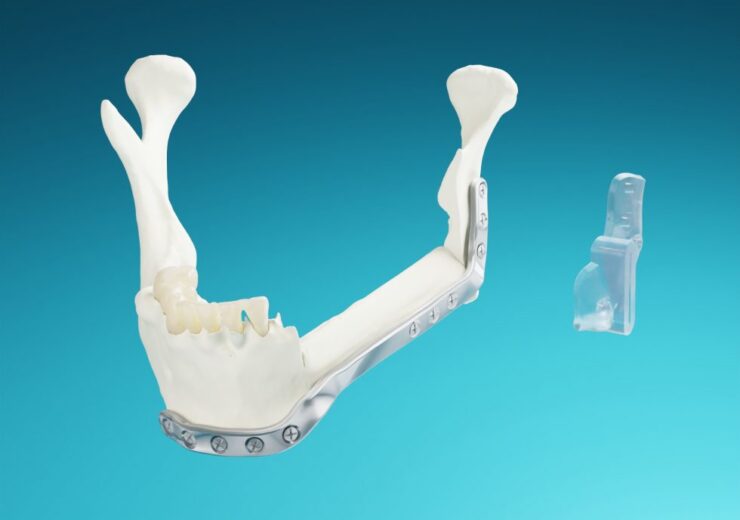
AccuPlate Patient-Specific Plates. (Credit: MedCAD.)
MedCAD has secured the US Food and Drug Administration (FDA) 510(k) approval for its AccuPlate patient-specific plates to aid mandibular reconstructive surgeries.
The Texas-based medical equipment manufacturer designed the plates based on the patient’s scanned anatomical data, and are milled out of pure, implant-grade titanium.
They are intended for use in mandibular reconstruction surgeries to stabilise bone after an osteotomy or traumatic event with or without bone grafts.
MedCAD said that the patient-matched design allows the plate to eliminate the need for bending, saving time in the operating room and optimising surgical outcomes.
Also, the AccuPlate product is designed to meet the needs of each patient and surgeon through a customisable mandible plating solution, said the company.
MedCAD president and CEO Nancy Hairston said: “We’re thrilled to bring AccuPlate to market. It’s a feature-dense product that fits perfectly in our portfolio of Custom Surgical Solutions.
“Our customized products are designed to help surgeons best meet the needs of individual patients, and AccuPlate does exactly that.”
Alongwith MedCAD’s FDA approved AccuPlan System, AccuPlate benefits from the entire surgical planning experience with guided fixation holes, osteotomies and positioning, the company said.
According to the company, surgeons can prescribe individual features like fixation hole placement and plate thickness, instead of graft placement in fibula free flap mandibular reconstruction.
Later, a custom surgical solution is reached, by optimising the design specifications of AccuPlate plates to work with guides and splints.
Established in 2007, MedCAD is engaged in providing cranial implants, patient-matched plating, surgical planning, and 3D modeling products and services.
MedCAD executive vice president Brian Buss said: “Years of intense product development work are clearly paying off with the 510(k) approval of AccuPlate, and our foray into titanium milling is an exciting new venture.
“Our singular goal is to meet our customers’ needs through new materials, new processes and new products and services.
“AccuPlate is a compelling new product garnering significant distribution partnership opportunities and driving forward our vision of Restoring Humanity One Patient at a Time.”
