The PMD-200 device is designed for monitoring nociception, and NOL leverages a multi-parametric sensor platform and advanced AI algorithms to convert complicated data into the patient’s Signature of Pain
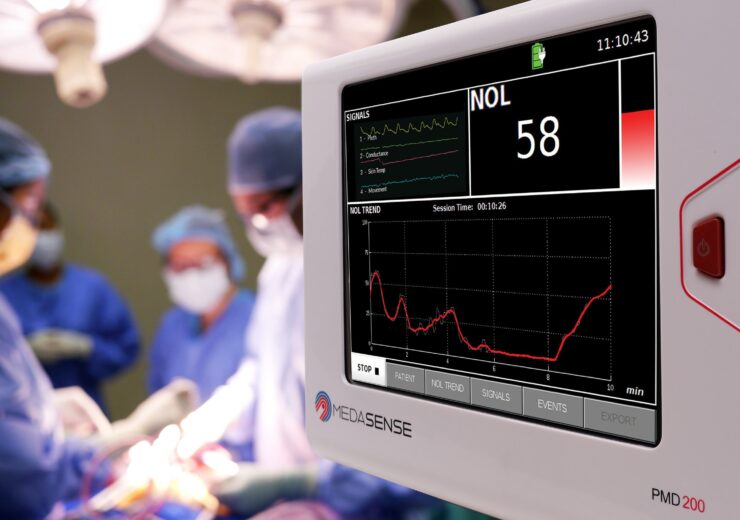
The FDA authorised PMD-200 monitor with NOL technology. (Credit: PRNewswire/Medasense Biometrics Ltd.)
Medasense Biometrics has received the US Food and Drug Administration (FDA) marketing authorisation for its PMD-200 patient monitor with NOL technology.
The US health agency granted the marketing authorisation under its De Novo premarket review pathway.
The Israeli medical equipment manufacturer developed the PMD-200 device for monitoring nociception, the physiological response to pain.
NOL leverages a multi-parametric sensor platform and advanced AI algorithms to convert complicated data into the patient’s Signature of Pain.
The technology is currently used in operating rooms and high-acuity settings, where patients are under anaesthesia and unable to communicate.
It enables clinicians to personalise pain management, control pain and prevent overmedication, said the company.
Medasense founder and CEO Galit Zuckerman-Stark said: “NOL technology has the potential to improve the lives of hundreds of millions of patients worldwide, and we have already seen the impact it has had in multiple countries.
“We are proud to offer the first and only measure of intraoperative pain (nociception) in the US, and to be able to offer meaningful innovation in the area of pain management to help patients undergoing surgery experience improved pain-related outcomes.”
The FDA approval is supported by clinical data that showed NOL-guided intraoperative analgesia resulted in improved postoperative pain scores in adult patients.
According to the company, NOL is the first and only monitoring technology authorised for measuring changes in nociception in adults receiving opioid or opioid sparing analgesics.
In the studies, intraoperative NOL monitoring has been shown to reduce postoperative pain for patients in the post anaesthesia care unit, and potentially reduced costs of care.
Charleston, South Carolina-based anaesthesiologist Frank Overdyk said: “The anaesthesia community has needed a technology like NOL for a long time.
“We have devices that monitor the depth of anaesthesia, we have TOF cuffs to check for patient movement, but the missing piece of the puzzle is a way to monitor the effect of the opioid or opioid sparing analgesia.
“This technology as an adjunctive to clinical judgment will provide a window into the patient’s nociceptive state during surgery so we can personalise the way we administer analgesia, improving the patient’s recovery.”
