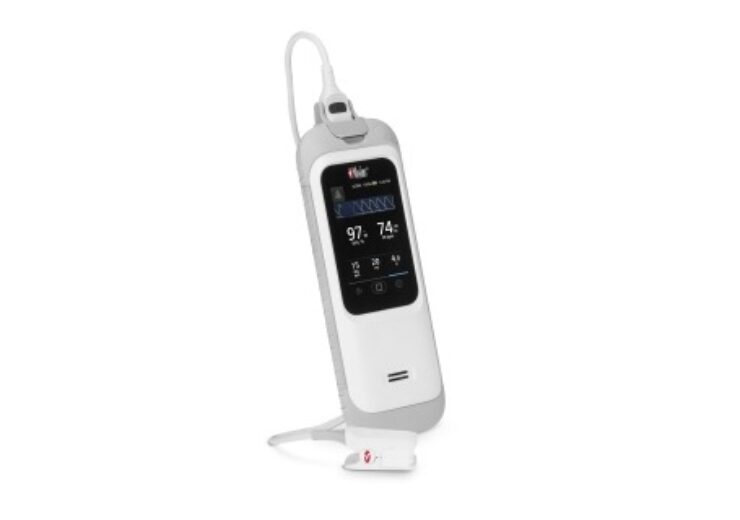
Rad-G Pulse Oximeter is a rugged handheld device providing SET pulse oximetry, respiration rate from the pleth (RRp), and other parameters
Masimo Rad-G Pulse Oximeter. (Credit: Business Wire.)
Masimo, a provider of non-invasive patient monitoring devices, has received FDA approval for its Rad-G Pulse Oximeter, which facilitates both spot-checking and continuous monitoring.
Rad-G Pulse Oximeter is a rugged handheld device that provides clinically verified SET pulse oximetry, respiration rate from the pleth (RRp), and other vital parameters.
Masimo said that the device can be used in different settings, including physicians’ offices, outpatient services, long-term care facilities, wellness clinics, first-response scenarios, and limited-resource environments.
Rad-G includes a long-lasting rechargeable battery, rubber casing, and a new direct-connect sensor capable of pulse oximetry or vital sign monitoring in both adults and children, said the company.
Also, the light weight, compact, portable device enables clinicians to rapidly assess patients and make informed care decisions.
Masimo founder and CEO Joe Kiani said: “With the Rad-G Pulse Oximeter, we set out to create an accessible, high-quality care solution that clinicians can rely on in a multitude of care settings.
“We are bringing our expertise in pulse oximetry to a smaller, more lightweight, rugged, and versatile handheld device, without sacrificing any of the advantages that help provide clinicians with critical insights into patient status.
“As the Covid-19 pandemic continues, it’s more imperative than ever that caregivers have access to the most accurate and reliable pulse oximetry monitoring technologies. We are proud to offer Rad-G among our suite of solutions powered by the breakthrough technology, SET pulse oximetry.”
Rad-G Pulse Oximeter also includes a new direct-connect Rad-G Sensor
The device also includes a new direct-connect Rad-G Sensor, which facilitate monitoring of both adult and paediatric patients, and eliminates the need for carrying different types of sensors.
In addition to the new sensor, Rad-G can be used with Masimo’s portfolio of reusable and single-patient-use sensors, enabling clinicians customise the solution.
Initially developed in partnership with The Bill & Melinda Gates Foundation as a spot-check device, the Rad-G device expands the capabilities of its predecessor with additional alarms feature.
The rechargeable battery of Rad-G facilitates 24 hours of use, allowing clinicians to work in outdoor, transport, and emergency scenarios.
The high-resolution screen of the device enables a continuous display of pleth waveform and the audible alarms will alert clinicians based on changes in patient status.
