Masimo and Mdoloris Medical Systems announced the CE marking of the Mdoloris Analgesia Nociception Index (ANI) module for the Masimo Root Patient Monitoring and Connectivity Hub, the first commercially available result of a Masimo Open Connect (MOC) third-party partnership, between Mdoloris and Masimo.
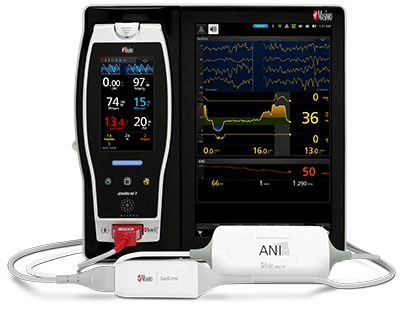
Image: Masimo Root with next generation SedLine brain function monitoring and Mdoloris ANI. Photo: courtesy of Masimo.
Masimo’s unique approach to medical technology integration through Masimo Open Connect partnerships addresses some of the major barriers to new technology adoption in patient monitoring.
The Root platform’s open architecture and advanced connectivity enable third-party companies to bypass barriers and the time it takes for traditional multi-parameter monitor integration by controlling their own Root integration projects.
Third parties can then independently develop, obtain regulatory approvals, and commercialize their own external MOC-9 module or MOC-Capp for Root using Masimo’s MOC software development kit and support from Masimo’s engineering and distribution teams.
Joe Kiani, Founder and CEO of Masimo, said, “We are proud to announce ANI, the first commercially available third-party MOC-9 module for Root, the first of many to come. With the ongoing expansion of its capabilities, Root becomes a more powerful bedside platform than ever. We believe that Root with Masimo Open Connect can do for patient monitoring what the PC did for computing: speed up the patient monitoring innovation cycle, reduce the cost of patient monitoring, and prolong the useful life of the equipment hospitals invest in.”
“We are delighted to be able to announce that ANI is now cleared for sale in the EU,” said Fabien Pagniez, Founder and CEO of Mdoloris Medical Systems. “We consider Masimo to be the most innovative company in the patient monitoring space and we believe Root offers a unique and compelling solution for implementing our ANI technology.”
ANI on Masimo Root has not received 510(k) clearance and is not available for sale in the United States.
Source: Company Press Release
