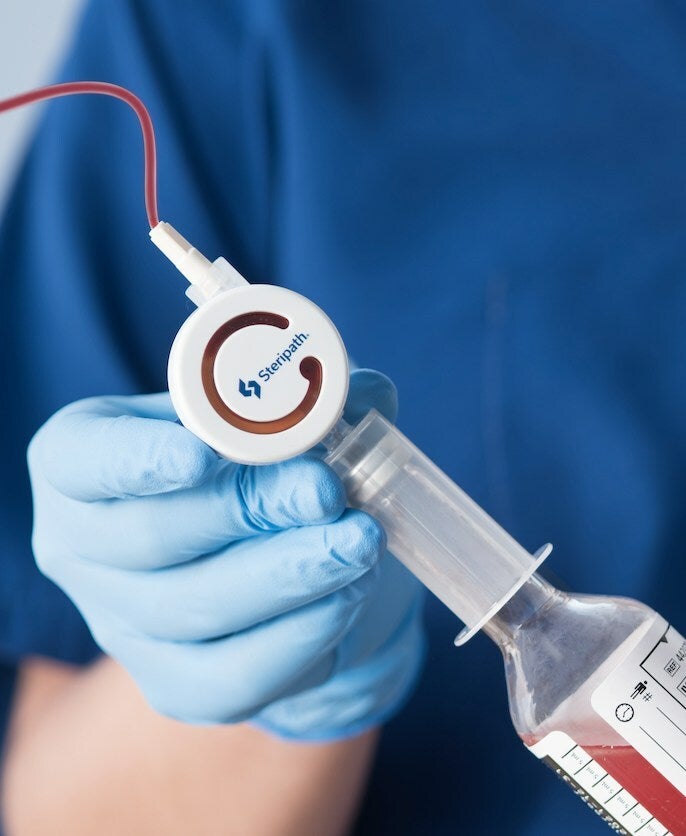
Magnolia Medical Technologies has received the US Food and Drug Administration (FDA) 510(k) approval for 19 new configurations within its Steripath Micro Initial Specimen Diversion Device (ISDD) platform.
Steripath Micro devices employ the same ISDD technology as Steripath ISDD products.
They feature a small, highly intuitive design for optimal user experience, with needle configurations integrating the BD Vacutainer UltraTouch push-button blood collection set.
UltraTouch needles have an ultra-thin-wall cannula that provides a larger inner diameter while maintaining a true-to-gauge size outer cannula diameter, known as BD RightGauge technology.
In addition, the BD PentaPoint cannula technology is integrated into BD Vacutainer UltraTouch wingsets to help penetrate the skin with greater ease.
Steripath Micro is the only low-diversion volume blood culture collection device platform approved in the US, to reduce blood culture contamination, said the medical device company.
The FDA approval is said to provide hospitals with new options, including direct-to-bottle and BD Vacutainer UltraTouch push-button blood collection set configurations.
Magnolia Medical CEO Greg Bullington said: “We are delighted to launch the expanded family of Steripath Micro configurations as an integral part of our Initial Specimen Diversion Device portfolio.
“We developed the Steripath Micro platform in close collaboration with our customers to ensure the ability to provide improved blood culture accuracy for all patient populations, including those that are most vulnerable.”
Magnolia Medical developed the Steripath Micro to optimise the blood culture collection process by allowing for fast, easy, and effective diversion and test sample collection.
Steripath Micro diverts the initial 0.5 to 1 ml of blood into the diversion chamber to prevent the potential contaminants from distorting the blood sample to be tested.
Upon completion of the diversion, the clinician can separate potential contaminants and automatically open a second, sterile blood flow pathway to collect blood, by pushing a button.
In October last year, BD and Magnolia Medical announced a commercial agreement to help US hospitals reduce blood culture contamination by improving sepsis testing accuracy.
Under the partnership, the companies jointly sold Steripath and Steripath Micro ISDD platforms, complementing BD Vacutainer push button and BD Vacutainer UltraTouch blood collection sets.
Bullington added: “The availability of these configurations will further accelerate our co-selling and co-marketing activities with BD as all Steripath Micro needle configurations come standard with BD’s best-in-class UltraTouch needle innovation.
“This new product offering demonstrates our continued commitment to providing hospitals and healthcare systems with the best possible solutions that optimally balance ease of use and clinical performance to support the achievement of CLSI and CDC’s new 1% goal for blood culture contamination rates.”




