US-based Lumos Diagnostics has merged with RPS Diagnostics for the creation of a full service development company.
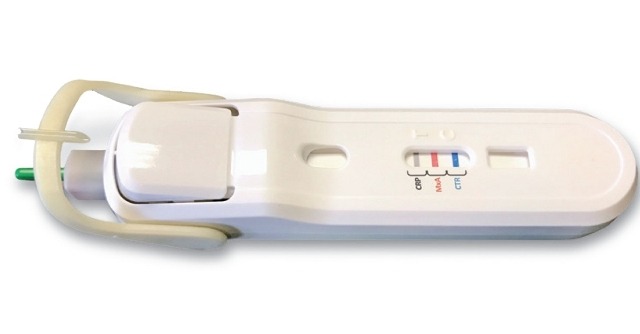
Image: FebriDx test is a rapid and in-office POC test. Photo: courtesy of Business Wire.
Lumos Diagnostics is a California-based full-service point-of-care (POC) diagnostic development company, while RPS Diagnostics is a Florida-based commercial diagnostic developer, manufacturer, and marketer of POC diagnostic tests.
Lumos Diagnostics is the name of the combined company, which will initially focus on the global launch of FebriDx test.
FebriDx test is a rapid and in-office POC test, which integrates a built-in safety lancet to obtain a fingerstick blood sample, rotating blood collection and transfer system and integrated push-button buffer activation, helping clinicians to quickly assess the body’s immune response to an acute respiratory infection (ARI).
The single use test detects patients within 10 minutes that have a clinically significant underlying infection, and helps to differentiate viral and bacterial ARIs through the simultaneous detection of both Myxovirus resistance protein A (MxA) and C-reactive protein (CRP) directly from peripheral whole blood.
FebriDx test already secured Health Canada approval, Saudi FDA clearance, Singapore Health Sciences Authority registration, as well as CE marking in Europe. It is not yet secured approval from the US Food and Drug Administration (FDA).
Lumos Diagnostics board chairman Sam Lanyon said: “We have evaluated many technologies over the years, and believe that combining RPS Diagnostics’ novel biomarker technology and commercial experience together with Lumos Diagnostics’ reader-based platform results in a highly strategic and synergistic union that will support a robust pipeline and commercial success.”
The diagnostic test solutions offered by Lumos Diagnostics use advanced digital reader platforms to assist healthcare professionals precisely diagnose and manage diseases and medical conditions.
