ivWatch, a provider of continuous monitoring devices for the early detection of intravenous (IV) infiltrations and extravasations, has secured medical device license (MDL) from Health Canada to sell the ivWatch Model 400 to Canadian health care organizations.
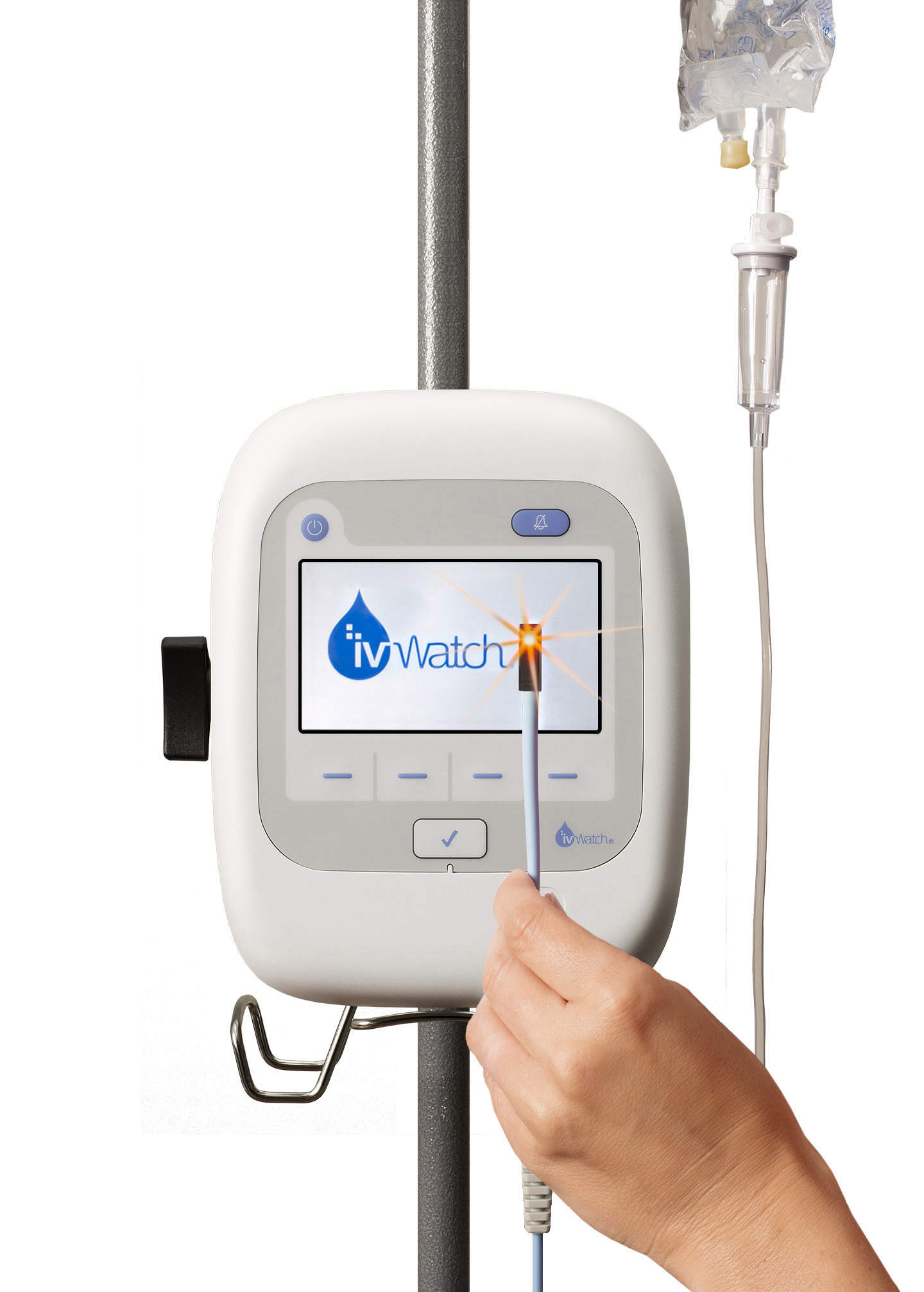
Image: ivWatch was issued a medical device license (MDL) by Health Canada permitting the company to sell the ivWatch Model 400 to Canadian health care organizations. Photo: Courtesy of Business Wire.
ivWatch sales and business development vice president Scott Hensley said: “This is yet another milestone allowing more medical care providers and patients access to a first-of-its-kind technology to minimize harm from peripheral IV infiltration/extravasation events (PIVIEs).
“Canada is one of the most progressive health care markets in the world and acknowledge PIVIEs as serious safety events. Clinicians understand the need for surveillance monitoring to improve patient safety.”
Every infiltration is a medication dosing and delivery error that can impact the patient through prolonged hospital stays, temporary harm or permanent injuries. Beyond the patient safety impact, any of these adverse events may result in increased expenses, significant legal risk and reputation damage for health care providers and facilities.
Hensley said: “We’re excited to work with health systems and pediatric hospitals who have quality improvement measures in place and stand ready to implement technology capable of drastically reducing the time to detect a PIVIE beyond what is currently possible by visual and tactile assessment.”
The MDL is the final step for Canadian market entry and was preceded by the company’s achievement of ISO 13485 Certification, the global standard for medical device quality management systems (QMS) developed by the International Organization for Standardization (ISO).
Source: Company Press Release
