Iradimed said that its 3883 MRI-compatible invasive blood pressure module is now available as an option to the 3880 MRI-compatible patient vital signs monitor.
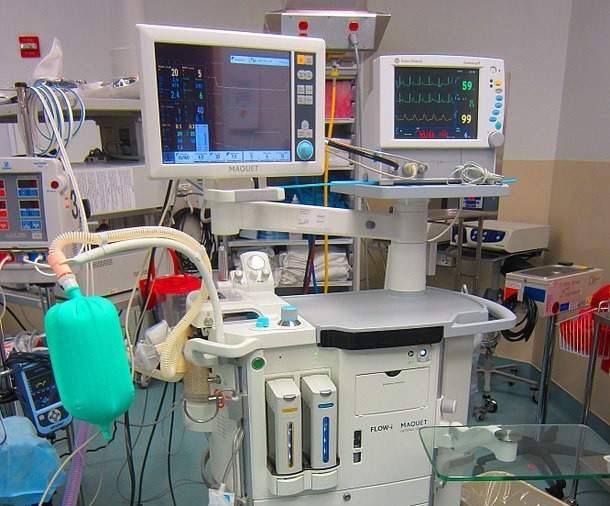
The company designed the 3880 MRI-compatible patient vital signs monitoring system using non-magnetic components and other special features that enable safe and accurate monitoring of vital signs of a patient, during various MRI procedures.
The patient monitoring system is claimed to have been designed to be easy-to-use, allowing for the effective communication of patient vital signs information to clinicians.
Iradimed president and CEO Roger Susi said: “This newly cleared device represents a feature extension to the 3880 MRI-compatible patient monitoring system, which we launched in the US just over a year ago. With the invasive blood pressure module, we now offer advanced monitoring capabilities to our customers.”
Susi continued saying: “Customers can now provide a more comprehensive suite of vital signs monitoring while transporting patients from hospital critical care departments to the MRI scanner room and back to critical care while maintaining continuous vital signs monitoring.”
The Iradimed 3880 MRI-compatible vital signs monitoring system’s compact and lightweight design enables uninterrupted monitoring during transport, resulting in increased patient safety and decreasing the amount of time critically ill patients are away from critical care units.
The vital signs monitoring system is claimed to be compact and lightweight, offering uninterrupted vital signs monitoring when patients are transporting from their critical care unit, to the MRI and back. This results in increased patient safety and decreases the amount of time critically ill patients are away from critical care units.
The 3880 system includes wireless ECG with dynamic gradient filtering, wireless SpO2 using Masimo SET algorithms, non-magnetic respiratory CO2, non-invasive blood pressure, invasive blood pressure, patient temperature and Masimo multi-gas anesthetic agent unit that has a continuous Minimum Alveolar Concentration measurements.
In September, the company secured regulatory clearance from the Japanese Ministry of Health Labor & Welfare for the 3880 MRI compatible patient vital signs monitoring system.






