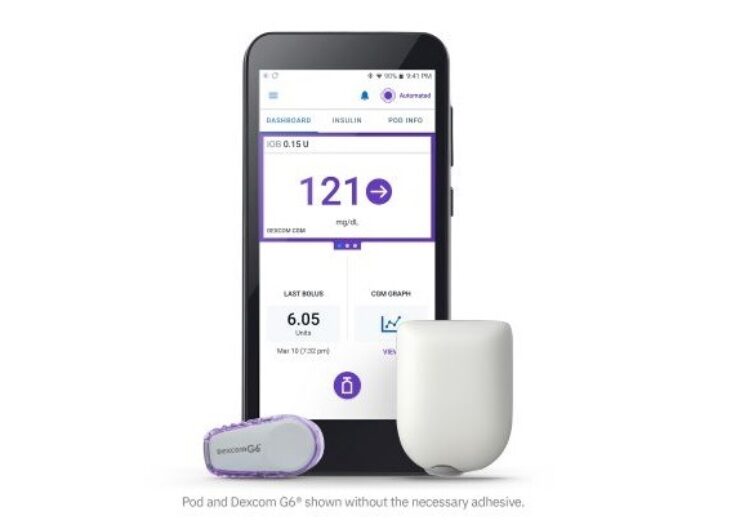
Omnipod 5 is a tubeless automated insulin delivery (AID) system that combines with the Dexcom G6 continuous glucose monitoring (CGM) system and a compatible smartphone
Omnipod 5 automated insulin delivery system. (Credit: Business Wire)
Insulet has secured approval from the US Food and Drug Administration (FDA) for its Omnipod 5 automated insulin delivery system for individuals aged six years and older with type 1 diabetes.
Omnipod 5 is claimed to be the first tubeless automated insulin delivery (AID) system, which combines with the Dexcom G6 continuous glucose monitoring (CGM) system and a compatible smartphone to automatically adjust insulin and provide protection against highs and lows.
The company has designed the Omnipod 5 to manage glucose without multiple daily injections, tubes and zero fingersticks.
Insulet president and CEO Shacey Petrovic said: “Omnipod 5 is a life-changing technology that we believe will revolutionize the market and the lives of people with diabetes.
“We are incredibly proud of this simple-to-use, elegant system, designed to deliver unmatched freedom and to greatly simplify insulin management and improve glucose control for our users.”
The system features tubeless pod enhanced with SmartAdjust technology, Dexcom G6 CGM and Omnipod 5 mobile app with its integrated SmartBolus calculator.
Insulet stated that an option is provided to the user to download the app on a compatible personal smartphone or use the Omnipod 5 controller that is provided free with the first prescription.
Upon receiving Dexcom CGM value and trend for every five minutes, SmartAdjust predicts glucose levels for the next 60 minutes, said the company.
Later, the system increases, decreases or pauses insulin delivery with the support of user’s desired and customised glucose target, thereby helping to provide protection against highs and lows.
Dexcom chairman, president and CEO Kevin Sayer said: “Omnipod 5 combines the accuracy and unmatched user experience of the Dexcom G6 CGM with the simplicity of tubeless insulin delivery to offer people with diabetes a revolutionary new way to optimize time in range.”
Insulet, which will launch Omnipod 5 through the pharmacy channel, is initially planning a limited market release of the product.
In August last year, Insulet announced the FDA approval for use of Eli Lilly’s Lyumjev (insulin lispro-aabc injection) 100 units/mL with its Omnipod products.
