The ALICE cardiopulmonary bypass device will go through a verification and validation phase before the firm plans to submit 510(k) clearance to the FDA
Inspira Technologieshas started the manufacturing process for the ALICE CPB device. (Credit: PRNewswire/Yakir Shukrun)
Inspira Technologies has started the manufacturing process for the ALICE CPB (cardiopulmonary bypass) device to support its plan of submitting the 510(k) clearance to the US Food and Drug Administration (FDA) in 2023.
The Israel-based respiratory support technology firm said that the ALICE CPB device will go through the verification and validation phase prior before pursuing FDA clearance.
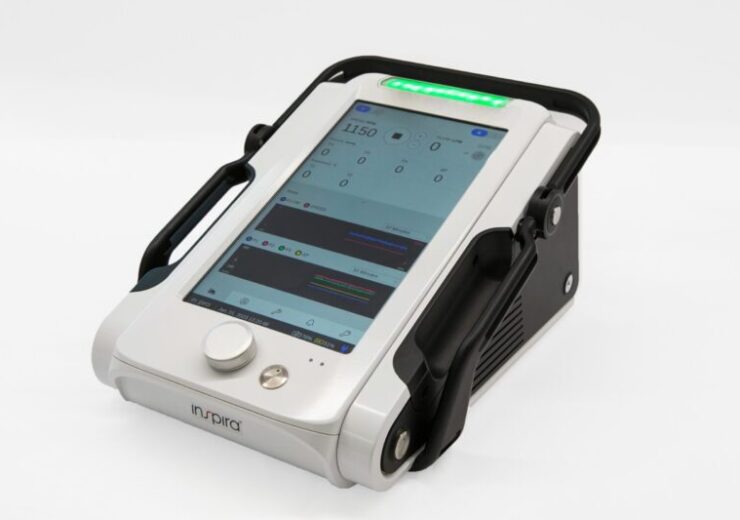
According to Inspira Technologies, the ALICE gadget is designed to be used in clinical environments that demand CPB support. The device is claimed to have a large touchscreen and a graphical interface to improve the visibility and usability of data displayed to medical staff.
It is designed to be lightweight as well as durable and is equipped with long battery life, said the company.
Inspira Technologies revealed that the ALICE CPB device is being developed by an end-to-end solution provider that delivers new product introduction (NPI) services to mass production capabilities for medical electronic device firms.
The contract given to the undisclosed firm covers full turnkey manufacturing, system integration, production of printed circuit board (PCB), assembly services, testing, and packaging in facilities that adhere to Good Manufacturing Practices (GMP) standards.
Inspira Technologies has plans to make ALICE the first device that can integrate the HYLA Blood Sensor. The sensor is designed to be non-invasive, make continuous measurements, and notify physicians in real time of changes in a patient’s blood indicators.
Inspira Technologies chief operating officer Avi Shabtai said: “Today, we believe that we have achieved a very important milestone in line with our strategy towards the production and delivery of Inspira Technologies’ products to the market.”
Upon FDA approval, the device production line will be extended for low-rate initial production (LWRIP). The new operational stage will help to develop infrastructure for serial manufacturing, quality control, and shipping.
For the planned future first deployments of the ALICE devices in the US and Israel, more units are anticipated to be manufactured, the respiratory technology firm added.
In July this year, the company signed an agreement with Glo-Med Networks for the distribution of the HYLA blood sensor device and disposable units across six states in the US.
