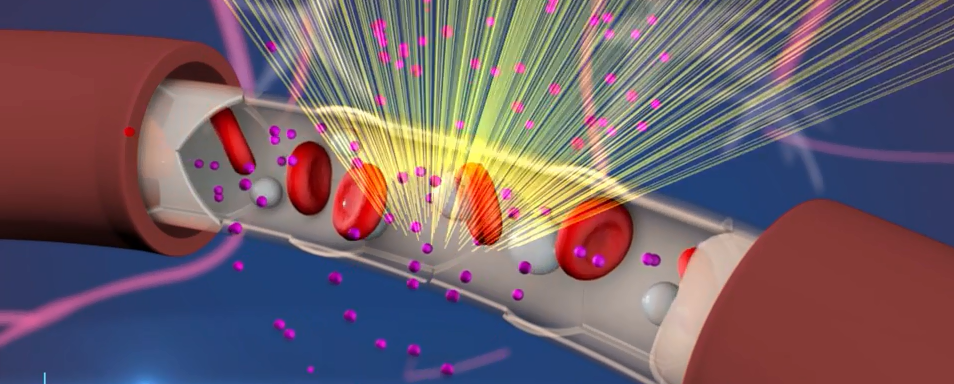
West Virginia University Rockefeller Neuroscience Institute executive chair Ali Rezai said: “It is exciting to lead this pioneering study to evaluate focused ultrasound for Alzheimer’s disease. The potential for blood brain barrier (BBB) disruption to aid in the clearance of beta-amyloid holds promise for developing new and innovative treatments that are crucial in the battle against Alzheimer’s and other neurodegenerative diseases.”
The study is a prospective, multi-center, single-arm study to evaluate the safety and efficacy of using INSIGHTEC’s Exablate Neuro low-frequency focused ultrasound to disrupt the BBB in patients diagnosed with AD.
INSIGHTEC CEO and board chairman Maurice R. Ferré said: “INSIGHTEC is spearheading clinical research by collaborating with leading researchers to advance brain health. We remain sharply focused on making a profound impact on the health and lives of people across America and around the globe.”
An early trial using the company’s Exablate Neuro to temporarily disrupt the BBB has been completed on five patients with early stage AD at Sunnybrook Health Sciences Centre in Toronto, Canada. The results by lead investigator, Nir Lipsman, were published in Nature Communications on 25 July, 2018.
Source: Company Press Release



