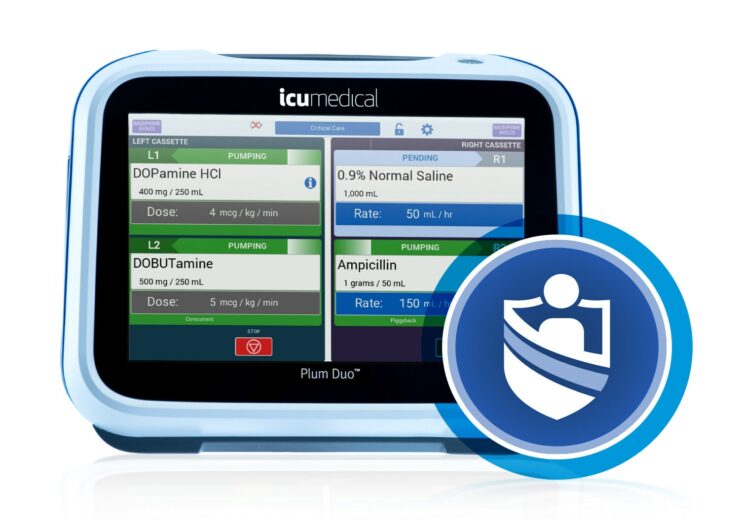
Plum Duo pump comes with a large colour touchscreen, intuitive user interface, and two channels to deliver up to four compatible IV medications, while LifeShield software provides advanced new tools for complete drug library management
Plum Duo infusion pump and LifeShield infusion safety software. (Credit: PRNewswire/ICU Medical, Inc)
ICU Medical has received the US Food and Drug Administration (FDA) 510(k) approval for its Plum Duo infusion pump with the LifeShield infusion safety software.
The Plum Duo pump is the latest addition to the company’s portfolio of infusion devices and builds on the accurate delivery of unique cassette technology used in its Plum 360 device.
It comes with a large colour touchscreen, a highly intuitive user interface, and two channels that deliver up to four compatible IV medications.
The LifeShield Infusion Safety Software is a unified cloud-based software suite, designed to provide advanced new tools for complete drug library management.
It enhances the clinicians’ ability to access, process and rapidly share information across an entire enterprise or health system to promote collaboration.
The US-based medical device maker plans to commercialise the Plum Duo infusion pump and LifeShield software in the US in early 2024.
ICU Medical Infusion Systems corporate vice president and general manager Dan Woolson said: “Receiving FDA clearance for the Plum Duo pump and LifeShield software is the first step in realising ICU Medical’s long-term vision of bringing customers best-of-breed devices with a shared platform and user experience.
“We are proud of this milestone and look forward to bringing our next-generation Medfusion and CADD products to the LifeShield platform, creating the most complete, precise, and technologically advanced infusion systems in the industry for our customers and their patients.”
ICU Medical said that its Plum Duo infusion pump and LifeShield infusion safety software, as a large-volume infusion delivery platform, will help improve the delivery of IV medication.
The system’s screen is equipped with guided touchscreen programming that differentiates important infusion parameters and alarms to provide near and far views of all parameters.
Its two channels with four lines will help enhance responsiveness in rapidly changing environments and reduce the footprint in high-acuity areas.
Plum Duo deploys a unique pumping mechanism for accurate and precise medication delivery, and PlumSet cassettes feature the company’s Clave needle-free connectors to reduce infection.
The system also features an in-line air trap that reduces air-in-line alarms and a cloud-based IV informatics solution with centralised drug library management and reporting.
ICU Medical said that its LifeShield software comes with an engaging user interface, which facilitates easy and effective content entry, drug library management and collaboration.
Its simplified drug library and firmware deployment are managed from a central location, to ensure devices are consistently updated and pumps are on the most current software version.
In addition to the Plum Duo infusion pump with LifeShield software, ICU Medical’s portfolio includes Plum 360, Medfusion 4000, CADD-Solis, and CADD-Solis VIP pumps.
