HTL-STREFA's intent is to ensure all caregivers providing finger-stick (capillary blood) testing for COVID-19 antibodies understand how best to perform the procedure to ensure high-quality samples while minimizing patient pain and discomfort
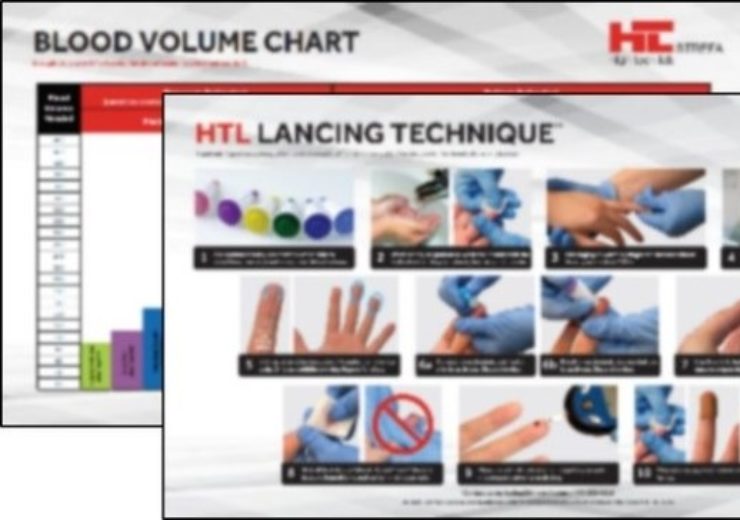
HTL-STREFA is providing best practice lancing technique to aid in COVID-19 antibody testing. (Credit: PRNewswire)
HTL-STREFA, the world-wide leader and inventor of the first safety lancet, announced today that they are making available their proprietary best practice for proper lancing technique to all front-line caregivers combating COVID-19.
HTL-STREFA’s intent is to ensure all caregivers providing finger-stick (capillary blood) testing for COVID-19 antibodies understand how best to perform the procedure to ensure high-quality samples while minimizing patient pain and discomfort. To support education of the technique, HTL-STREFA is making available an illustrated step-by-step copyrighted poster outlining their lancing technique.
“In our experience, we have seen caregivers use varying methods in lancing with their patients,” said Michael Billedo, Safety Marketing Director for HTL-STREFA, Inc. “We thought there would be strong interest amongst caregivers if we consolidated these best practices from their peers into a simple step-by-step process.
“We then consulted with the Center for Phlebotomy Education (www.phlebotomy.com) to validate that these instructions are safe and effective,” Mr. Billedo continues. “For example, we were intrigued to learn specific fingers and locations are preferred for sampling. Also, we were further surprised to learn that squeezing or ‘milking’ the location after pricking is not encouraged as it risks introducing fluid that could compromise the sample quality and results.”
Having invented the safety lancet in 1994, HTL-STREFA has become a global reference and preferred partner for capillary blood sampling and safety medical sharps. The company’s safety lancets are designed and proven to obtain various blood volumes measured in microliters (µL) for various assays, inclusive of COVID-19, and are available for immediate delivery.
Source: Company Press Release
