The Edison System is designed to offer a non-invasive method to mechanically destroy liver tumours, including the partial or complete destruction of unresectable liver tumours through histotripsy
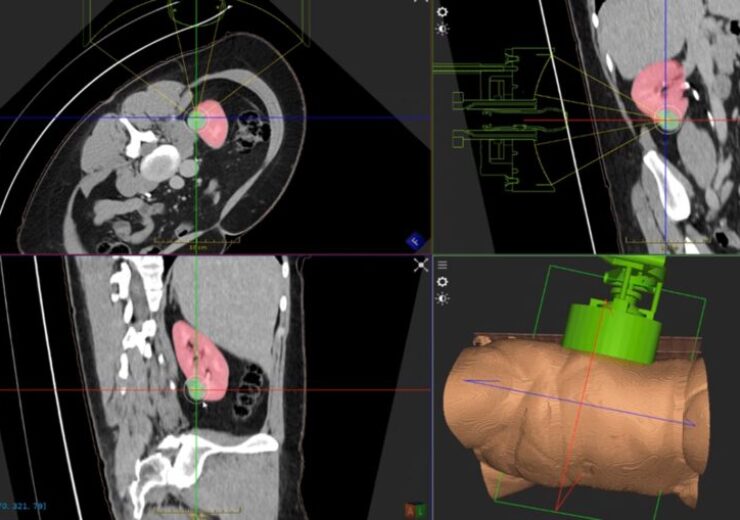
An illustration of HistoSonics technology targeting kidney tissue. (Credit: Business Wire)
Medical device company HistoSonics has treated the first kidney tumour patient in the #HOPE4KIDNEY trial of its breakthrough Edison Histotripsy System.
The Edison System is designed to offer a non-invasive method to mechanically destroy liver tumours, including the partial or complete destruction of unresectable liver tumours through histotripsy.
This investigational device trial was approved by the US Food and Drug Administration (FDA) last year.
#HOPE4KIDNEY is designed to assess the safety and efficacy of the Edison System to non-invasively eliminate targeted kidney tumours without using any needles or incisions.
The multi-centre, open-label, single-arm trial will recruit up to 68 patients.
The procedure was completed by urologist Michael McDonald at AdventHealth Celebration in Osceola County under the direction of AdventHealth Research Institute.
HistoSonics president and CEO Mike Blue said: “We are extremely excited to have Dr McDonald and his team at AdventHealth Celebration treat the first patient as part of the #HOPE4KIDNEY Trial.
“Our goal is to enable physicians to precisely target and destroy kidney tumours with our novel, noninvasive solution, avoiding the morbidity and complications seen with current invasive surgery or ablative techniques.”
The image-guided sonic beam therapy system from HistoSonics delivers non-invasive, customised treatments with accuracy and control using advanced imaging and in-house sensing technologies.
It mechanically destroys targeted tissue and liquifies them at sub-cellular levels using controlled acoustic cavitation, generated from focused sound energy.
The medical device company claims that its technology offers significant advantages to patients, including the treatment site’s capacity for rapid healing and resorption.
The HistoSonics platform is said to offer doctors the capability of monitoring the destruction of tissue under continuous real-time visualisation and control, which is not possible in existing modalities.
The company plans to use key learnings from its initial Phase 1 kidney study, called the CAIN Trial, and technical enhancements with its Edison platform during the #HOPE4KIDNEY trial.
In October last year, HistoSonics received the FDA marketing authorisation for the Edison System.
