HeartFlow Planner enables interventional cardiologists to model treatment strategies in real time before entering the catheterisation lab
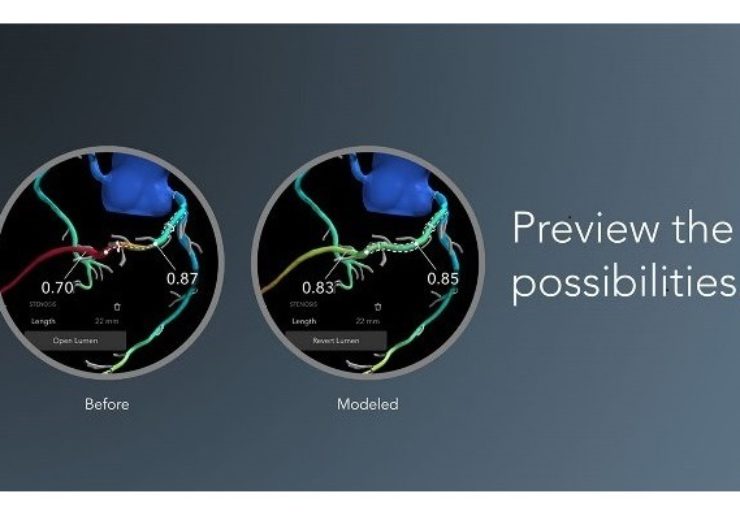
Image: HeartFlow Planner is a non-invasive and real-time virtual modelling tool for coronary artery disease (CAD) intervention. Photo: courtesy of Business Wire.
Digital health company HeartFlow has secured approval from the US Food and Drug Administration (FDA) for its non-invasive and real-time virtual modelling tool for coronary artery disease (CAD) intervention.
HeartFlow Planner will allow interventional cardiologists to virtually model clinical scenarios vessel-by-vessel and prepare treatment strategies for patients with CAD before each procedure.
HeartFlow Planner non-invasive and real-time virtual modelling tool
The new virtual modelling tool will also help interventional cardiologists assess cases with colleagues and provide a clear picture of the initial treatment plan.
HeartFlow Planner, which is a pre-procedure planning tool, is based on an idealised model of the HeartFlow Analysis, a colour-coded 3D model of a patient’s coronary arteries.
Physicians can apply this model to detect blockages and explore multiple treatment scenarios by virtually modifying the vessel.
The non-invasive tool will allow physicians to understand the impact of each modeled treatment strategy in real time.
Data from a patient’s non-invasive coronary computed tomography angiogram (CTA) will be uploaded from the hospital’s system to HeartFlow’s software application working in the AWS cloud.
HeartFlow Analysis will apply advanced computer algorithms to address millions of complex equations to simulate blood flow and evaluate the impact of blockages on coronary blood flow.
An idealised coronary anatomy model and physiology simulation created from the HeartFlow Analysis will be used by the HeartFlow Planner. The physicians can detect stenoses and virtually modify the vessel.
HeartFlow Planner will show the modified FFRct values in real time for each treatment scenario to understand the impact of the modeled treatment strategy.
Focal stenoses, serial stenoses, and borderline cases are the common scenarios for using HeartFlow Planner.
HeartFlow president and CEO Dana G. Mead, Jr said: “As part of the company’s commitment to improve the standard of care for patients with coronary artery disease, the FDA clearance of HeartFlow Planner represents a major milestone in ensuring more physicians have access to our innovative healthcare solution.
“We look forward to introducing the HeartFlow Planner to the interventional cardiology community and continuing to change the way coronary artery disease is diagnosed and treated.”
In March 2017, HeartFlow had partnered with Siemens Healthineers to develop a solution that has the potential to reduce cardiac care costs.
