The approval was based on data collected from the pivotal stage of the Gore Assured clinical study, which showed 100% closure success at the six month evaluation in patients with a successful implant.
The study assessed the safety and efficacy of ASD closure using the Gore Cardioform ASD occluder in 125 patients with evidence of right heart volume overload showing the need for defect closure.
Gore’s study recruited patients between the ages of 2 and 84, across 22 investigation sites, including 15 children’s hospitals.
According to the company, the pivotal study achieved its safety, closure, and technical success primary endpoints.
Assured study co-principal investigator Dr Matthew Gillespie said: “The FDA approval of the GORE CARDIOFORM ASD Occluder is a significant milestone for innovation in the minimally invasive treatment of ASDs.
“This soft, conformable device was not previously available for this range of defects but is now an option for larger defects that typically have a greater risk for complications, including right heart enlargement, atrial fibrillation, and pulmonary hypertension.”
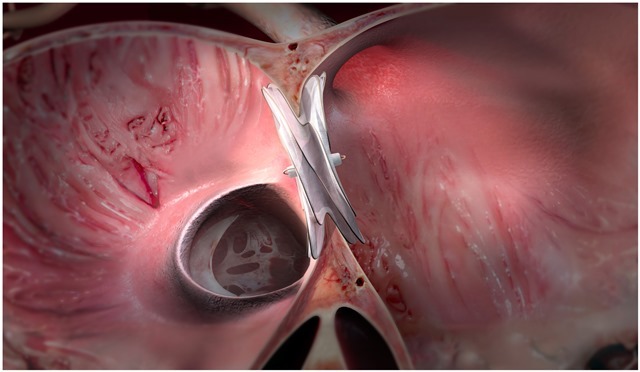
Gore Cardioform ASD occluder’s anatomically adaptable waist is said to conform to the defect to close ASDs from 8mm to 35mm in diameter, including those without a retro-aortic rim, by facilitating optimal tissue ingrowth, while maintaining thromboresistance.
The occluder portfolio is also comprised of Cardioform septal occluder for ASD closure for defects up to 17mm. It received FDA approval in 2018 for patent foramen ovale (PFO) closure to prevent recurrent ischemic stroke.
Cardioform septal occluder approval was based on data from the Reduce clinical study, which showed the safety and efficacy of PFO closure with a Gore device plus antiplatelet therapy compared against antiplatelet therapy alone in patients with a PFO and history of cryptogenic stroke.
Gore structural heart pipeline leader Dr Jake Goble said: “We developed the GORE CARDIOFORM ASD Occluder in partnership with leading interventional cardiologists around the globe, and its design is informed by decades of experience in technological innovation and dedication to improving patient care.”



